Key Concepts
By the end of this section, you will be able to do the following:
- Discuss the importance of electrons in the transfer of energy in living systems
- Explain how ATP is used by cells as an energy source
Energy production within a cell involves many coordinated chemical pathways. Most of these pathways are combinations of oxidation and reduction reactions, which occur at the same time. An oxidation reaction strips an electron from an atom in a compound, and the addition of this electron to another compound is a reduction reaction. Because oxidation and reduction usually occur together, these pairs of reactions are called oxidation reduction reactions, or redox reactions.
Electrons and Energy
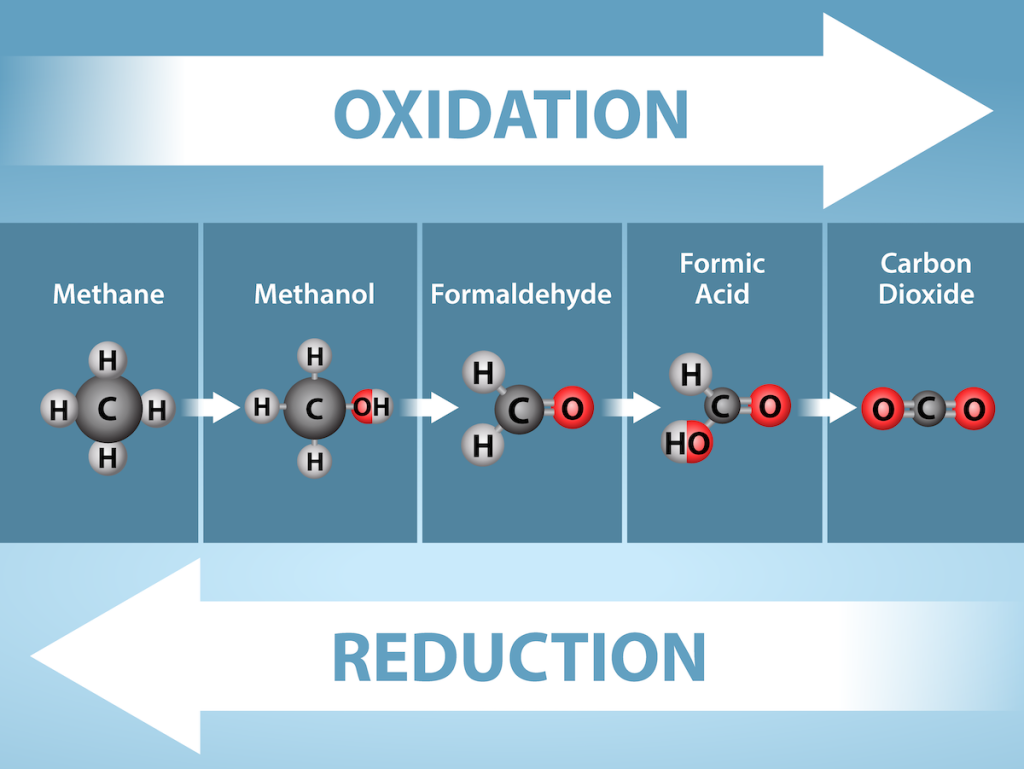
The removal of an electron from a molecule (oxidizing it), results in a decrease in potential energy in the oxidized compound. However, the electron (sometimes as part of a hydrogen atom) does not remain unbonded in the cytoplasm of a cell. Rather, the electron is shifted to a second compound, reducing the second compound. The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced compound). The transfer of electrons between molecules is important because most of the energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the form of high-energy electrons allows the cell to transfer and use energy in an incremental fashion—in small packages rather than in a single, destructive burst. This chapter focuses on the extraction of energy from food; you will see that as you track the path of the transfers, you are tracking the path of electrons moving through metabolic pathways.
Electron Carriers
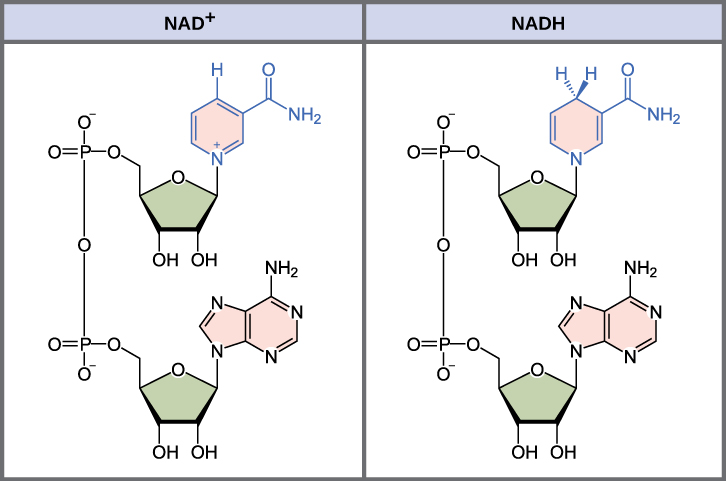
In living systems, a small class of compounds functions as electron shuttles: they bind and carry high-energy electrons between compounds in biochemical pathways. The principal electron carriers we will consider are derived from the B vitamin group and are derivatives of nucleotides. These compounds can be easily reduced (that is, they accept electrons) or oxidized (they lose electrons). Nicotinamide adenine dinucleotide (NAD) (Figure 7.3) is derived from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron). Note that if a compound has an “H” on it, it is generally reduced (e.g., NADH is the reduced form of NAD).
NAD+ can accept electrons from an organic molecule according to the general equation:
When electrons are added to a compound, it is reduced. A compound that reduces another is called a reducing agent. In the above equation, RH is a reducing agent, and NAD+ is reduced to NADH. When electrons are removed from a compound, it is oxidized. A compound that oxidizes another is called an oxidizing agent. In the above equation, NAD+ is an oxidizing agent, and RH is oxidized to R.
Similarly, flavin adenine dinucleotide (FAD+) is derived from vitamin B2, also called riboflavin. Its reduced form is FADH2. A second variation of NAD, NADP, contains an extra phosphate group. Both NAD+ and FAD+ are extensively used in energy extraction from sugars, and NADP plays an important role in anabolic reactions and photosynthesis in plants.
ATP in Living Systems
A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would result in excessive thermal motion that could damage and then destroy the cell. Rather, a cell must be able to handle that energy in a way that enables the cell to store energy safely and release it for use only as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP). ATP is often called the “energy currency” of the cell, and, like currency, this versatile compound can be used to fill any energy need of the cell. How? It functions similarly to a rechargeable battery.
When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. The energy is used to do work by the cell, usually when the released phosphate binds to another molecule, thereby activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to move the contractile muscle proteins. Recall the active transport work of the sodium-potassium pump in cell membranes. ATP alters the structure of the integral protein that functions as the pump, changing its affinity for sodium and potassium. In this way, the cell performs work, pumping ions against their electrochemical gradients.
ATP Structure and Function
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to a ribose molecule and to a single phosphate group (Figure 7.4). Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
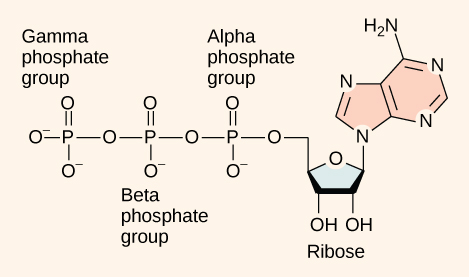
The addition of a phosphate group to a molecule requires energy. Phosphate groups are negatively charged and thus repel one another when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of one or two phosphate groups from ATP, a process called dephosphorylation, releases energy.
Energy from ATP
Hydrolysis is the process of breaking complex macromolecules apart. During hydrolysis, water is split, or lysed, and the resulting hydrogen atom (H+) and a hydroxyl group (OH–), or hydroxide, are added to the larger molecule. The hydrolysis of ATP produces ADP, together with an inorganic phosphate ion (Pi), and the release of free energy. To carry out life processes, ATP is continuously broken down into ADP, and like a rechargeable battery, ADP is continuously regenerated into ATP by the reattachment of a third phosphate group. Water, which was broken down into its hydrogen atom and hydroxyl group (hydroxide) during ATP hydrolysis, is regenerated when a third phosphate is added to the ADP molecule, reforming ATP.
Obviously, energy must be infused into the system to regenerate ATP. Where does this energy come from? In nearly every living thing on Earth, the energy comes from the metabolism of glucose, fructose, or galactose, all isomers with the chemical formula C6H12O6 but different molecular configurations. In this way, ATP is a direct link between the limited set of exergonic pathways of glucose catabolism and the multitude of endergonic pathways that power living cells.
Phosphorylation
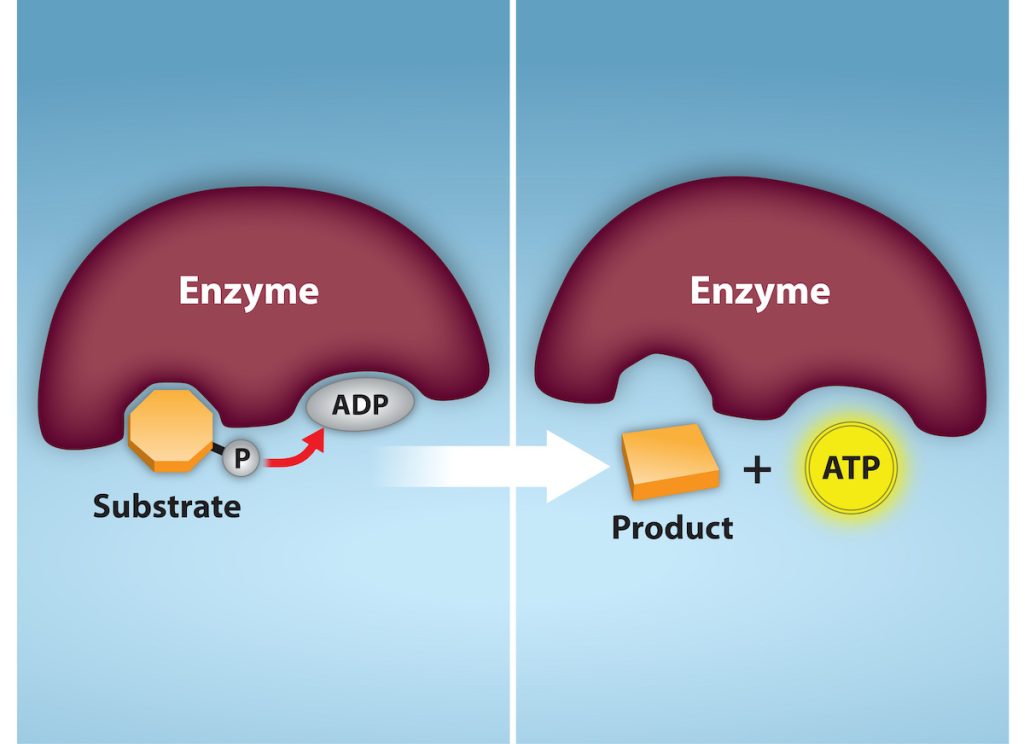
Recall that, in some chemical reactions, enzymes may bind to several substrates that react with each other on the enzyme, forming an intermediate complex. An intermediate complex is a temporary structure, and it allows one of the substrates (such as ATP) and reactants to more readily react with each other; in reactions involving ATP, ATP is one of the substrates and ADP is a product. During an endergonic chemical reaction, ATP forms an intermediate complex with the substrate and enzyme in the reaction. This intermediate complex allows the ATP to transfer its third phosphate group, with its energy, to the substrate, a process called phosphorylation. Phosphorylation refers to the addition of the phosphate (~P). This is illustrated by the following generic reaction, in which A and B represent two different substrates:
When the intermediate complex breaks apart, the energy is used to modify the substrate and convert it into a product of the reaction. The ADP molecule and a free phosphate ion are released into the medium and are available for recycling through cell metabolism.
Substrate Phosphorylation
ATP is generated through two mechanisms during the breakdown of glucose. A few ATP molecules are generated (that is, regenerated from ADP) as a direct result of the chemical reactions that occur in the catabolic pathways. A phosphate group is removed from an intermediate reactant in the pathway, and the free energy of the reaction is used to add the third phosphate to an available ADP molecule, producing ATP (Figure 7.5). This very direct method of phosphorylation is called substrate-level phosphorylation.
Oxidative Phosphorylation
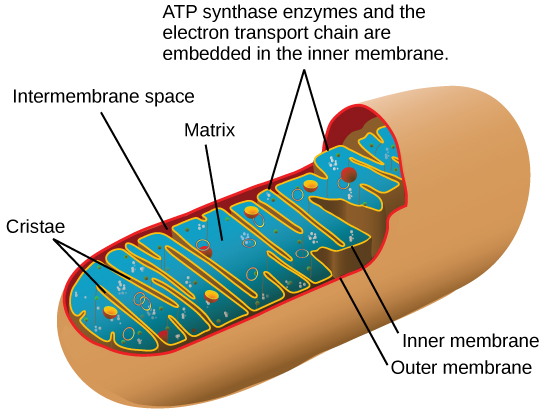
Most of the ATP generated during glucose catabolism, however, is derived from a much more complex process, chemiosmosis, which takes place in mitochondria (Figure 7.6) within a eukaryotic cell or the plasma membrane of a prokaryotic cell. Chemiosmosis, a process of ATP production in cellular metabolism, is used to generate 90 percent of the ATP made during glucose catabolism and is also the method used in the light reactions of photosynthesis to harness the energy of sunlight. The production of ATP using the process of chemiosmosis is called oxidative phosphorylation because of the involvement of oxygen in the process.
Career Connection
Mitochondrial Disease Physician
What happens when the critical reactions of cellular respiration do not proceed correctly? This may happen in mitochondrial diseases, which are genetic disorders of metabolism. Mitochondrial disorders can arise from mutations in nuclear or mitochondrial DNA, and they result in the production of less energy than is normal in body cells. In type 2 diabetes, for instance, the oxidation efficiency of NADH is reduced, impacting oxidative phosphorylation but not the other steps of respiration. Symptoms of mitochondrial diseases can include muscle weakness, lack of coordination, stroke-like episodes, and loss of vision and hearing. Most affected people are diagnosed in childhood, although there are some adult-onset diseases. Identifying and treating mitochondrial disorders is a specialized medical field. The educational preparation for this profession requires a college education, followed by medical school with a specialization in medical genetics. Medical geneticists can be board certified by the American Board of Medical Genetics and go on to become associated with professional organizations devoted to the study of mitochondrial diseases, such as the Mitochondrial Medicine Society and the Society for Inherited Metabolic Disorders.

