Key Concepts
By the end of this section, you will be able to do the following:
- Understand how the cell cycle is controlled by mechanisms that are both internal and external to the cell
- Explain how the three internal “control checkpoints” occur at the end of G1, at the G2/M transition, and during metaphase
- Describe the molecules that control the cell cycle through positive and negative regulation
The length of the cell cycle is highly variable, even within the cells of a single organism. In humans, the frequency of cell turnover ranges from a few hours in early embryonic development, to an average of two to five days for epithelial cells, and to an entire human lifetime spent in G0 by specialized cells, such as cortical neurons or cardiac muscle cells.
There is also variation in the time that a cell spends in each phase of the cell cycle. When rapidly dividing mammalian cells are grown in a culture (outside the body under optimal growing conditions), the length of the cell cycle is about 24 hours. In rapidly dividing human cells with a 24-hour cell cycle, the G1 phase lasts approximately nine hours, the S phase lasts 10 hours, the G2 phase lasts about four and one-half hours, and the M phase lasts approximately one-half hour. By comparison, in fertilized eggs (and early embryos) of fruit flies, the cell cycle is completed in about eight minutes. This is because the nucleus of the fertilized egg divides many times by mitosis but does not go through cytokinesis until a multinucleate “zygote” has been produced, with many nuclei located along the periphery of the cell membrane, thereby shortening the time of the cell division cycle. The timing of events in the cell cycle of both “invertebrates” and “vertebrates” is controlled by mechanisms that are both internal and external to the cell.
Regulation of the Cell Cycle by External Events
Both the initiation and inhibition of cell division are triggered by events external to the cell when it is about to begin the replication process. An event may be as simple as the death of nearby cells or as sweeping as the release of growth-promoting hormones, such as human growth hormone (HGH or hGH). A lack of HGH can inhibit cell division, resulting in dwarfism, whereas too much HGH can result in gigantism. Crowding of cells can also inhibit cell division. In contrast, a factor that can initiate cell division is the size of the cell: As a cell grows, it becomes physiologically inefficient due to its decreasing surface-to-volume ratio. The solution to this problem is to divide.
Whatever the source of the message, the cell receives the signal, and a series of events within the cell allows it to proceed into interphase. Moving forward from this initiation point, every parameter required during each cell cycle phase must be met or the cycle cannot progress.
Regulation at Internal Checkpoints
It is essential that the daughter cells produced be exact duplicates of the parent cell. Mistakes in the duplication or distribution of the chromosomes lead to mutations that may be passed forward to every new cell produced from an abnormal cell. To prevent a compromised cell from continuing to divide, there are internal control mechanisms that operate at three main cell-cycle checkpoints: A checkpoint is one of several points in the eukaryotic cell cycle at which the progression of a cell to the next stage in the cycle can be halted until conditions are favorable. These checkpoints occur near the end of G1, at the G2/M transition, and during metaphase (Figure 10.11).
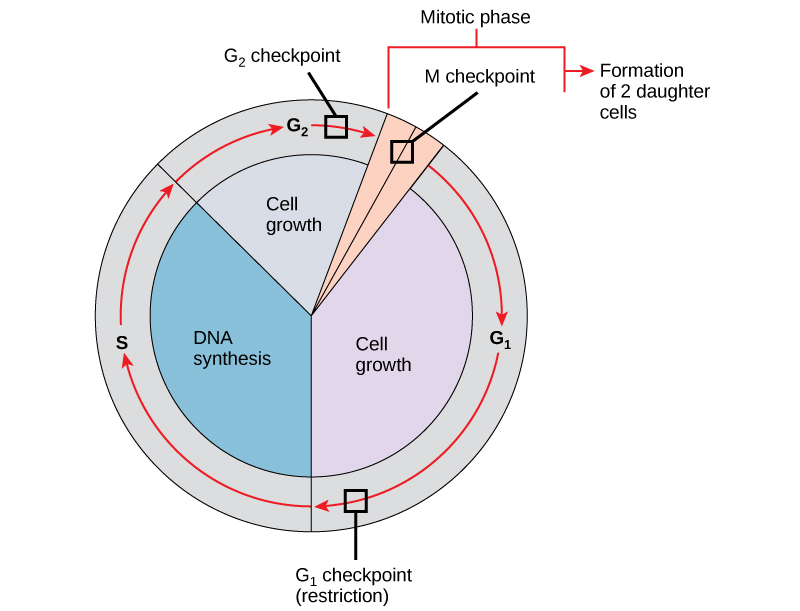
The G1 Checkpoint
The G1 checkpoint determines whether all conditions are favorable for cell division to proceed. The G1 checkpoint, also called the restriction point (in yeast), is a point at which the cell irreversibly commits to the cell division process. External influences, such as growth factors, play a large role in carrying the cell past the G1 checkpoint. In addition to adequate reserves and cell size, there is a check for genomic DNA damage at the G1 checkpoint. A cell that does not meet all the requirements will not be allowed to progress into the S phase. The cell can halt the cycle and attempt to remedy the problematic condition, or the cell can advance into G0 and await further signals when conditions improve.
The G2 Checkpoint
The G2 checkpoint bars entry into the mitotic phase if certain conditions are not met. As at the G1 checkpoint, cell size and protein reserves are assessed. However, the most important role of the G2 checkpoint is to ensure that all of the chromosomes have been replicated and that the replicated DNA is not damaged. If the checkpoint mechanisms detect problems with the DNA, the cell cycle is halted, and the cell attempts to either complete DNA replication or repair the damaged DNA.
The M Checkpoint
The M checkpoint occurs near the end of the metaphase stage of karyokinesis. The M checkpoint is also known as the spindle checkpoint, because it determines whether all the sister chromatids are correctly attached to the spindle microtubules. Because the separation of the sister chromatids during anaphase is an irreversible step, the cycle will not proceed until the kinetochores of each pair of sister chromatids are firmly anchored to at least two spindle fibers arising from opposite poles of the cell.
Link to Learning
Regulator Molecules of the Cell Cycle
In addition to the internally controlled checkpoints, there are two groups of intracellular molecules that regulate the cell cycle. These regulatory molecules either promote progress of the cell to the next phase (positive regulation) or halt the cycle (negative regulation). Regulator molecules may act individually, or they can influence the activity or production of other regulatory proteins. Therefore, the failure of a single regulator may have almost no effect on the cell cycle, especially if more than one mechanism controls the same event. However, the effect of a deficient or non-functioning regulator can be wide-ranging and possibly fatal to the cell if multiple processes are affected.
Positive Regulation of the Cell Cycle
Two groups of proteins, called cyclins and cyclin-dependent kinases (Cdks), are termed positive regulators. They are responsible for the progress of the cell through the various checkpoints. The levels of the four cyclin proteins fluctuate throughout the cell cycle in a predictable pattern (Figure 10.12). Increases in the concentration of cyclin proteins are triggered by both external and internal signals. After the cell moves to the next stage of the cell cycle, the cyclins that were active in the previous stage are degraded by cytoplasmic enzymes, as shown in Figure 10.12 below.
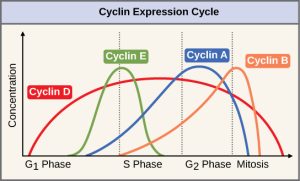
Cyclins regulate the cell cycle only when they are tightly bound to Cdks. To be fully active, the Cdk/cyclin complex must also be phosphorylated in specific locations to activate the complex. Like all kinases, Cdks are enzymes (kinases) that in turn phosphorylate other proteins. Phosphorylation activates the protein by changing its shape. The proteins phosphorylated by Cdks are involved in advancing the cell to the next phase. (Figure 10.13). The levels of Cdk proteins are relatively stable throughout the cell cycle; however, the concentrations of cyclin fluctuate and determine when Cdk/cyclin complexes form. The different cyclins and Cdks bind at specific points in the cell cycle and thus regulate different checkpoints.
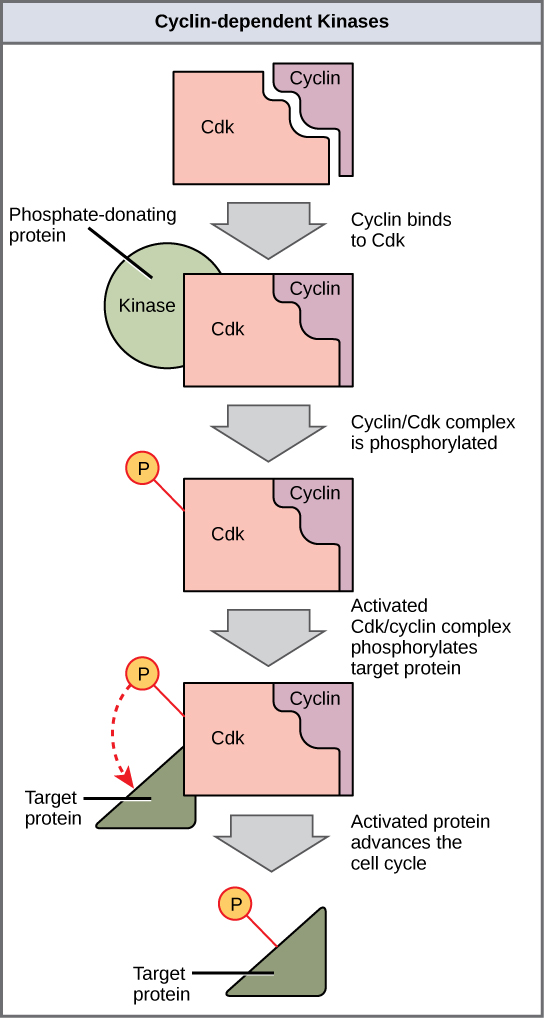
Because the cyclic fluctuations of cyclin levels are largely based on the timing of the cell cycle and not on specific events, regulation of the cell cycle usually occurs by either the Cdk molecules alone or the Cdk/cyclin complexes. Without a specific concentration of fully activated cyclin/Cdk complexes, the cell cycle cannot proceed through the checkpoints.
Although the cyclins are the main regulatory molecules that determine the forward momentum of the cell cycle, there are several other mechanisms that fine-tune the progress of the cycle with negative, rather than positive, effects. These mechanisms essentially block the progression of the cell cycle until problematic conditions are resolved. Molecules that prevent the full activation of Cdks are called Cdk inhibitors. Many of these inhibitor molecules directly or indirectly monitor a particular cell-cycle event. The block placed on Cdks by inhibitor molecules will not be removed until the specific event that the inhibitor monitors is completed.
Negative Regulation of the Cell Cycle
The second group of cell-cycle regulatory molecules are negative regulators, which stop the cell cycle. Remember that in positive regulation, active molecules cause the cycle to progress.
The best understood negative regulatory molecules are retinoblastoma protein (Rb), p53, and p21. Retinoblastoma proteins are a group of tumor-suppressor proteins common in many cells. We should note here that the 53 and 21 designations refer to the functional molecular masses of the proteins (p) in kilodaltons (a dalton is equal to an atomic mass unit, which is equal to one proton or one neutron or 1 g/mol). Much of what is known about cell-cycle regulation comes from research conducted with cells that have lost regulatory control. All three of these regulatory proteins were discovered to be damaged or non-functional in cells that had begun to replicate uncontrollably (i.e., became cancerous). In each case, the main cause of the unchecked progress through the cell cycle was a faulty copy of the regulatory protein.
Rb, p53, and p21 act primarily at the G1 checkpoint. p53 is a multi-functional protein that has a major impact on the commitment of a cell to division because it acts when there is damaged DNA in cells that are undergoing the preparatory processes during G1. If damaged DNA is detected, p53 halts the cell cycle and then recruits specific enzymes to repair the DNA. If the DNA cannot be repaired, p53 can trigger apoptosis, or cell suicide, to prevent the duplication of damaged chromosomes. As p53 levels rise, the production of p21 is triggered. p21 enforces the halt in the cycle dictated by p53 by binding to and inhibiting the activity of the Cdk/cyclin complexes. As a cell is exposed to more stress, higher levels of p53 and p21 accumulate, making it less likely that the cell will move into the S phase.
Rb, which largely monitors cell size, exerts its regulatory influence on other positive regulator proteins. In the active, dephosphorylated state, Rb binds to proteins called transcription factors, most commonly, E2F (Figure 10.14). Transcription factors “turn on” specific genes, allowing the production of proteins encoded by that gene. When Rb is bound to E2F, production of proteins necessary for the G1/S transition is blocked. As the cell increases in size, Rb is slowly phosphorylated until it becomes inactivated. Rb releases E2F, which can now turn on the gene that produces the transition protein, and this particular block is removed. For the cell to move past each of the checkpoints, all positive regulators must be “turned on,” and all negative regulators must be “turned off.”
Visual Connection
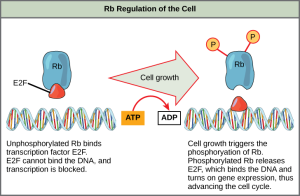
Rb and other proteins that negatively regulate the cell cycle are sometimes called tumor suppressors. Why do you think the name tumor suppressor might be appropriate for these proteins?

