6.4 Enzymes and Regulation of Metabolism
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Explain why enzymes are necessary to facilitate most biochemical reactions in cells by addressing the barrier of activation energy.
- Evaluate how the structure of enzymes is important for their function, and what factors can change or influence that structure.
- Compare and contrast the mechanisms that cells can use to regulate enzyme function, and therefore regulate the activity of metabolic pathways.
Now that we’ve discussed how cells manage energy transfers to support endergonic processes and reactions, let’s talk about how cells deal with the other barrier to chemical reactions: activation energy. Recall that every chemical reaction has an activation energy (EA) that must be overcome for the reaction to proceed (Chapter 6.1). Large activation energies cause reactions to proceed very slowly, regardless of whether those reactions are exergonic or endergonic. Most biochemical reactions must be facilitated by a catalyst, a substance that speeds up the reaction rate but is not consumed by the reaction. In biological system, those catalysts are called enzymes, and most of those enzymes are proteins. Enzymes are important for both facilitating chemical reactions, but also for regulating when and where those reactions happen. In this section, we will explore how enzymes make reactions more efficient, and how cells can use regulation of enzymes to ensure metabolism is appropriately controlled.
Mechanisms of Enzyme Function
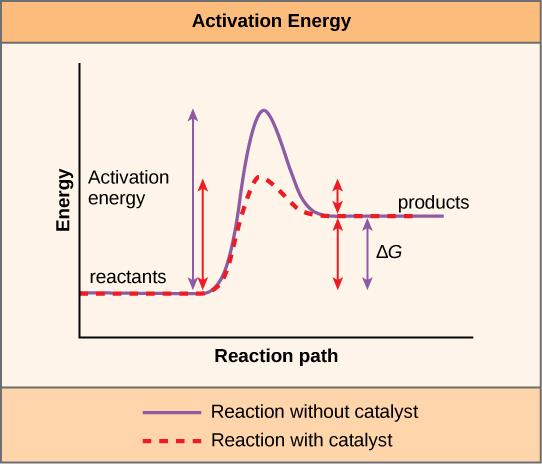
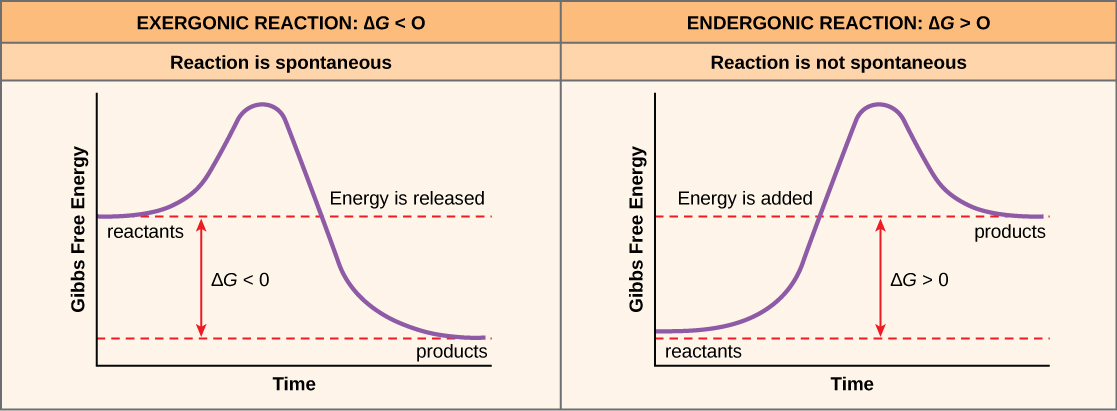
Catalysts (including enzymes) speed up chemical reaction rates by lowering the activation energy of a chemical reaction (Figure 6.15). Enzymes do this by creating an environment that lowers the energy of the transition state – a point in the chemical reaction that usually has high energy and low stability. It is important to remember that enzymes do not change a reaction’s ∆G; they do not change whether a reaction is exergonic (spontaneous) or endergonic because enzymes do not change the energy of the reactants or products (Figure 6.15). Enzymes are also not consumed by a chemical reaction so they can be used to catalyze the same reaction many times within a cell.
Cells contain many different enzymes, each of which catalyzes a specific reaction. To understand why enzymes are highly specific catalysts, we need to discuss enzyme structure and function. Most enzymes are proteins, and therefore are comprised of amino acids. Because there are so many (20) different amino acids that can be incorporated into proteins, there is a large variety in the amino acid composition of proteins within cells. Therefore, cells can make a large variety of enzymes, each with a different shape and function.
Enzyme Active Site and Substrate Specificity
The most important part of an enzyme’s shape is the part of the enzyme that binds to the chemical reactants, also called substrate(s). There may be one or more substrates, depending on the chemical reaction. In some reactions, a single-reactant substrate breaks down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products. The substrate may also be quite large, such as another protein.
The active site is the location within the enzyme where the substrates bind. This is where the “action” (chemical reaction) happens. Each enzyme has a unique combination of amino acid residues (also side chains, or R groups) within the active site. Different properties characterize each residue. These can be large or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral. The unique combination of amino acid residues, their positions, sequences, structures, and properties, creates a very specific chemical environment within the active site. This specific environment is suited to bind to a specific chemical substrate (or substrates), facilitating the specificity of enzymes. The “best fit” results from the attraction between the substrate(s) and the active site’s shape and the amino acid functional groups.
Induced Fit and Enzyme Function
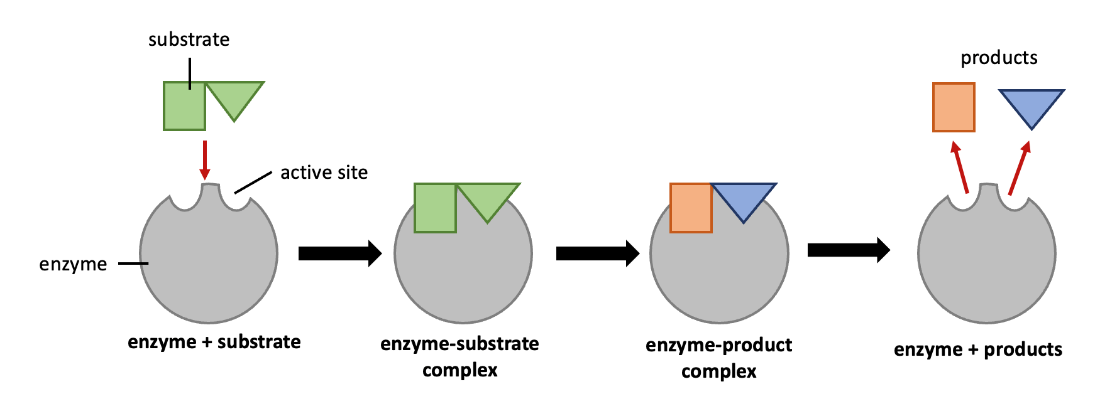
While the fit between enzyme and substrate is important, flexibility of enzyme structure is important for its catalytic properties – i.e., the ability of enzymes to lower the activation energy of a reaction. The induced fit model describes the dynamic interaction between enzyme and substrate (Figure 6.16). As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure, forming an enzyme-substrate complex. This enzyme-substrate complex has a slightly different shape than the enzyme alone, and this different shape creates an environment in the active site that helps lower of energy of the reaction’s transition state. By lowering the energy of the transition state, the enzyme lowers the reaction’s activation energy and promotes its rapid progression. Enzymes can also lower activation energies by (reversibly) taking part in the chemical reaction itself. The amino acid residues can provide certain ions or chemical groups that form covalent bonds with substrate molecules as a necessary step of the reaction process, but the enzyme is returned to its original state at the end of the chemical reaction. After an enzyme catalyzes a reaction, it releases its product(s) from the active site.
Enzyme Example: Amylase
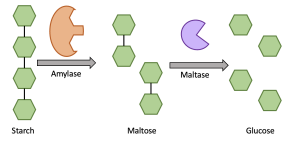
Amylase is an essential enzyme which allows for our bodies to digest and absorb carbohydrates as they are too large to be absorbed as they are. Amylase catalyses the breakdown of carbohydrates, such as starch, into smaller molecules. Specifically, amylase’s active site binds to starch (substrate), forming the enzyme/substrate complex, where amylase hydrolyzes the glycosidic bonds holding the carbohydrate together, breaking it into the smaller molecule maltose (product), which is then released by the enzyme. Maltose can be further broken down by maltase into glucose, which can be easily absorbed and used as a primary energy source for our body (Figure 6.17).
Link to Learning
Metabolism Control Through Enzyme Regulation
The metabolic needs of cells often change over time, so cells need to be able to control which metabolic pathways are active or inactive at any given time. Since the rates of biochemical reactions are controlled by activation energy, and enzymes lower and determine activation energies for chemical reactions, the relative amounts and functioning of the variety of enzymes within a cell ultimately determine which reactions will proceed and at which rates. Thus, the most effective way to modify metabolic activity is to modify the activity of key enzymes that catalyze steps of the relevant metabolic pathways. Anything that can modify protein structure can modify enzyme function, including environmental factors like pH and temperature, and the binding or presence of specific molecules.
Environmental Effects on Enzyme Activity
The fact that active sites are so perfectly suited to provide specific environmental conditions also means that they are subject to local environmental influences. It is true that increasing the environmental temperature generally increases reaction rates, enzyme-catalyzed or otherwise. However, increasing or decreasing the temperature outside of an optimal range of an enzyme can affect chemical bonds within the active site in such a way that they are less well suited to bind substrates, decreasing the chemical reaction rate (Figure 6.18). High temperatures will eventually cause enzymes, like other proteins, to denature, a process that changes the substance’s natural properties. Likewise, the local environment’s pH can also affect enzyme function. Active site amino acid residues have their own acidic or basic properties that are optimal for catalysis. These residues are sensitive to changes in pH that can impair the way substrate molecules bind. Enzymes are suited to function best within a certain pH range, and, as with temperature, extreme environmental pH values (acidic or basic) can cause enzymes to denature as well.
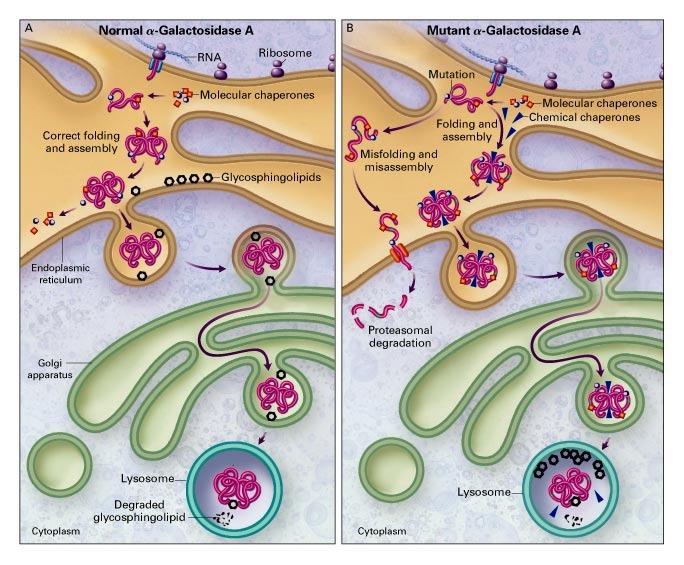
Enzyme Compartmentalization
For chemical reactions to be catalyzed, enzymes must be able to interact with their substrate(s). In eukaryotic cells, enzymes that support specific pathways are often compartmentalized into specific organelles that contain their substrates. For example, lysosomes contain macromolecules that must be broken down by the cell. The cell must move appropriate enzymes to the lysosome to support those metabolic processes, as shown in Figure 6.19. Conversely, cells can prevent the movement of enzymes to the location of their substrates to inhibit those metabolic processes.
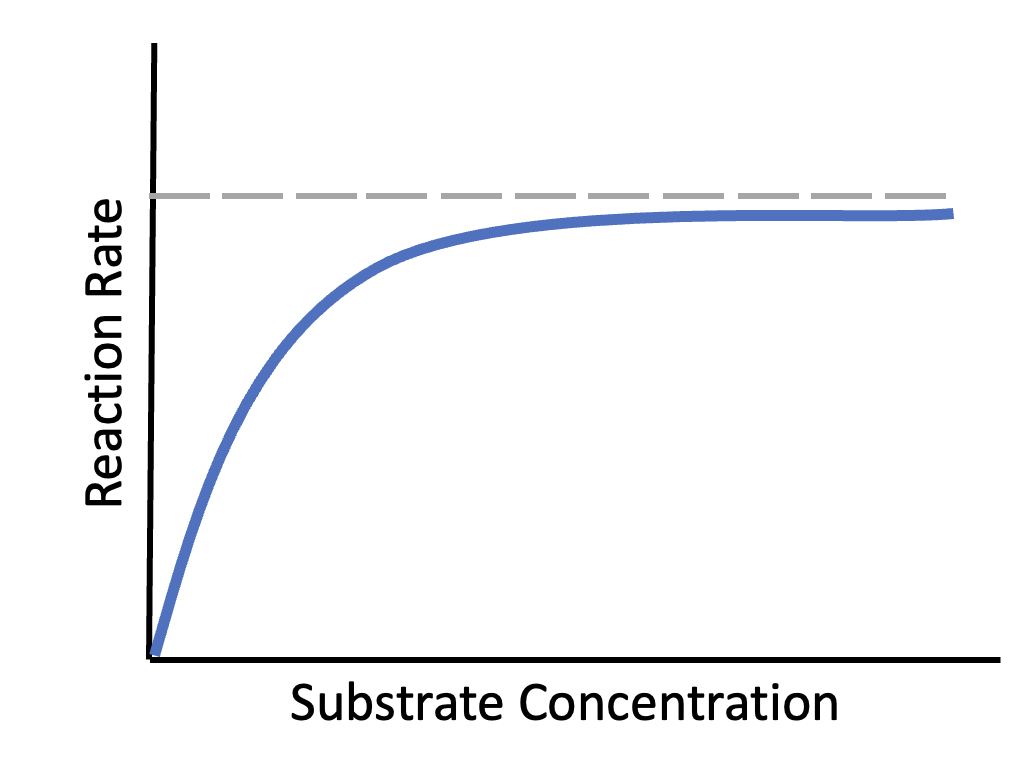
Substrate Availability
For a given concentration of active enzymes, as substrate concentration increases, reaction rate increases (Figure 6.20). The reaction rate will plateau (level out) when there are more substrate molecules present than can be catalyzed at one time by the enzymes – i.e., every enzyme processing substrates as quickly as possible, but there are still substrates “waiting” to be converted into products. For example, after eating a meal, cells in your body will experience an abundance of the substrate glucose. Consequently, reactions involving glucose, such as the first step of glycolysis, should be happening at a faster rate. If there is already a lot of the substrate present, shown by the plateau region in Figure 6.20, cells can only increase reaction rate further by increasing the amount of active enzyme. There are two ways this can happen: the cells can make more enzyme by transcribing and translating proteins, or they can activate existing enzymes (see next section).
Covalent Modifications
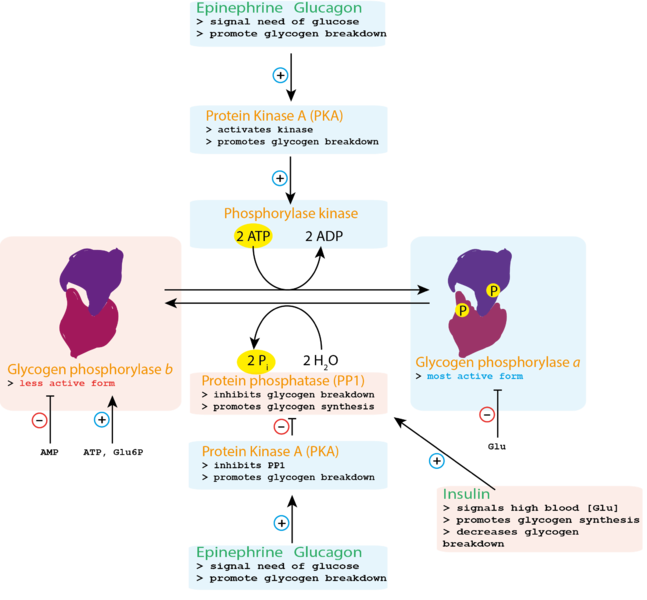
Cells can activate or inhibit existing enzymes via a number of mechanisms, including covalent modifications and interaction with inhibitor or activator molecules. Covalent modifications include attaching or detaching certain chemical groups to amino acids in the enzyme via covalent bonds. Let’s look at an example to see how this can work. The most well-studied example of covalent modification is phosphorylation: the addition of an inorganic phosphate group (PO4–; the same kind of group found in ATP) to amino acids that have hydroxyl groups (OH) in their R group, such as serine, threonine, or tyrosine. Other forms of covalent modification include acetylation (addition of an acetyl group, usually to a lysine) and prenylation (addition of a hydrocarbon side chain, most often to a cysteine side chain).
One example of an enzyme whose activity is regulated by phosphorylation is glycogen phosphorylase (see Figure 6.10) for the metabolic pathway it’s involved in). In human cells, glycogen phosphorylase can be phosphorylated at Ser14 (the 14th amino acid in the protein, which is a serine), causing in a change to the enzyme’s structure that increases its effectiveness at catalyzing chemical reactions. We call this more active form glycogen phosphorylase a (Figure 6.21), and the addition of the phosphate group is catalyzed by an enzyme. Glycogen phosphorylase can also undergo dephosphorylation (the removal of a that phosphate group), resulting in decreased enzyme activity. We call this less active form glycogen phosphorylase b (Figure 6.21), and the removal of the phosphate group is catalyzed by a different enzyme.
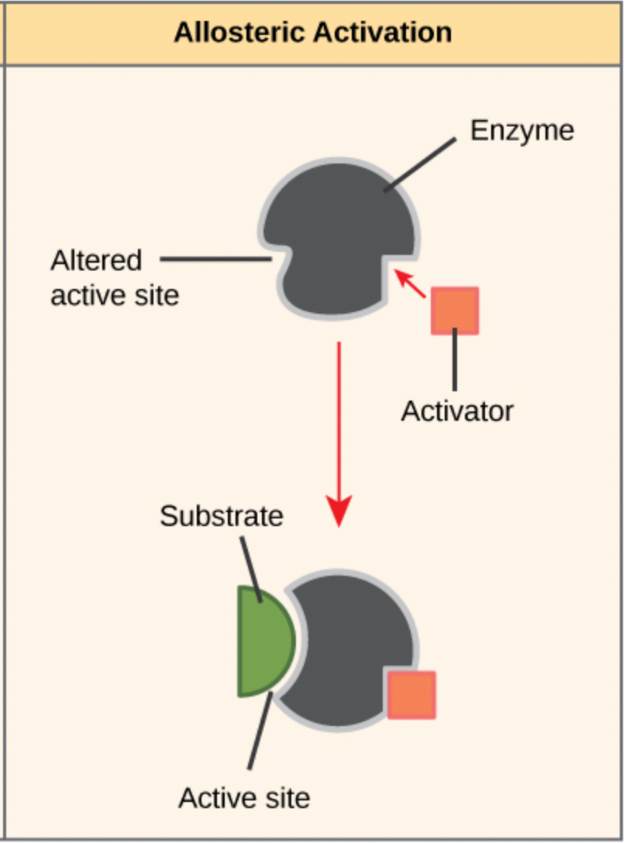
Enzyme Activators, Inhibitors, Cofactors, and Coenzymes
Cells can make or use molecules that activate enzyme activity, some of which bind to active sites, while others bind to allosteric sites. An allosteric site (“allo” = other) is any a region of the enzyme that is not the active site, and can bind to a molecule of some kind. Allosteric activators are regulatory molecules that bind to an enzyme’s allosteric site and induce a conformational change that increases the affinity of the enzyme’s active site for its substrate (Figure 6.22). Many enzymes don’t work optimally, or even at all, unless bound to other specific non-protein helper molecules, either temporarily through ionic or hydrogen bonds or permanently through stronger covalent bonds. Two types of helper molecules are cofactors and coenzymes, which often bind to the enzyme’s active site. Binding to these molecules promotes optimal conformation and function for their respective enzymes (Figure 6.23). They change the shape of the active site so the substrate can bind better to its respective enzyme. Cofactors are inorganic ions such as iron (Fe2+) and magnesium (Mg2+). Coenzymes are organic molecules (containing carbon and hydrogen) required for enzyme action.
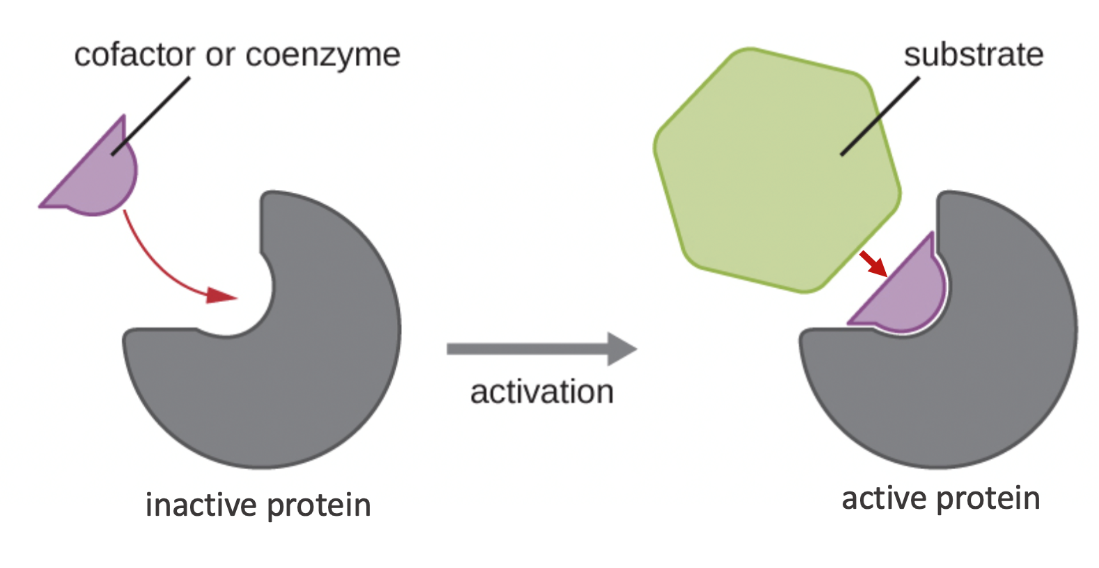
Most diets supply organisms with various cofactors and coenzymes needed in to regulate their enzyme function. One example of an enzyme that requires a cofactor is DNA polymerase, which requires a bound zinc ion (Zn2+) to build DNA molecules. The most common sources of coenzymes are dietary vitamins. Some vitamins are precursors to coenzymes and others act directly as coenzymes. Vitamin C, for example, is a coenzyme for multiple enzymes that take part in building the collagen in connective tissue.
Cells can also make or use molecules that inhibit enzyme activity, some of which bind to active sites, while others bind to allosteric sites. Competitive inhibition is when an enzyme inhibitor binds to the active site of an enzyme, thereby blocking the substrate from binding (Figure 6.24). Because the enzyme can no longer associate with the substrate properly, the rate of reaction decreases. This is shown by the green line in Figure 6.25. In non-competitive inhibition, an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site. Allosterically regulated enzymes can be composed of more than one polypeptide, meaning that they have more than one protein subunit. When an allosteric inhibitor binds to an enzyme, all active sites on the protein subunits change slightly such that they bind their substrates with less efficiency (Figure 6.24). This is shown by the blue line in Figure 6.25.
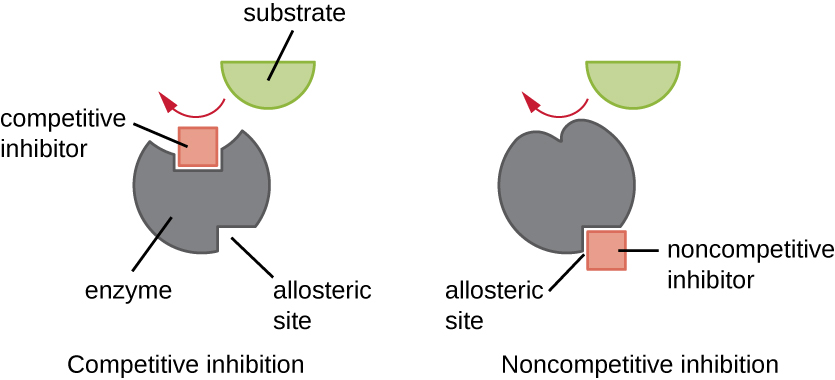
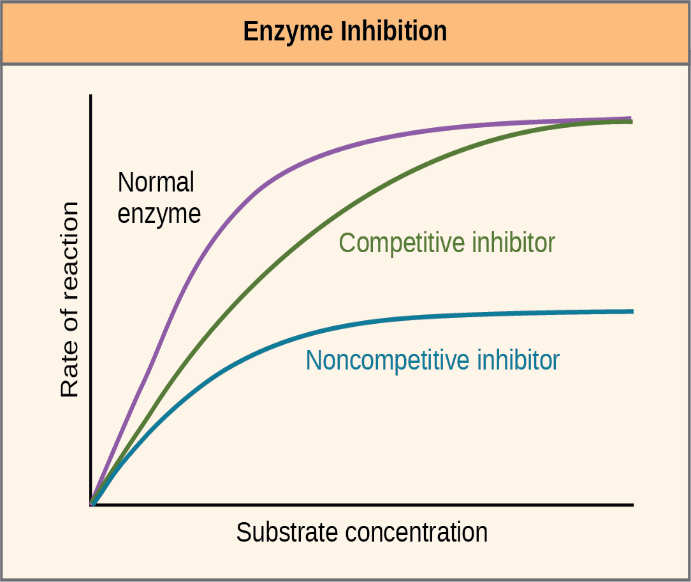
Inhibitors can often act as effective drugs or toxins. An example of a competitive inhibitor that kills bacteria is sulfanilamide (an antibacterial drug). Many bacteria need to synthesize folic acid, and one step in the metabolic pathway involves an enzyme called dihydropteroate synthase (DHPS). The normal substrate of DHPS (para-aminobenzoic acid; PABA) has some structural and chemical similarities to sulfanilamide. Sulfanilamide can competitively bind to the active site of DHPS, inhibiting synthesis of folic acid and bacterial growth. An example of a non-competitive inhibitor is cyanide, which binds to cytochrome oxidase (complex IV in Figure 6.14) in the mitochondrial electron transport system (ETS). Cyanide therefore prevents the catalytic activity of converting oxygen to water in the ETS, disrupting the synthesis of ATP in cellular respiration. Without a steady source of ATP, cells will die. Therefore, cyanide is an effective toxin in many organisms.
Feedback Inhibition in Metabolic Pathways
Perhaps the most relevant sources of enzyme regulatory molecules, with respect to cellular metabolism, are cellular metabolic reaction products themselves. In a most efficient and elegant way, cells have evolved to use their own reactions’ products for feedback inhibition of enzyme activity. Feedback inhibition involves using a reaction product to regulate its own further production. The cell responds to the abundance of specific products by slowing down its production via metabolic pathways. Such reaction products may inhibit the enzymes that catalyzed their production through the mechanisms (e.g., allosteric regulation) that we described above.
In the example in Figure 6.26, feedback inhibition occurs when the pathway’s end product (isoleucine) inhibits (indicated by red bar) an enzyme earlier in the pathway. In this example, isoleucine will bind to an allosteric site on the enzyme threonine deaminase and prevent threonine from binding to this enzyme’s active site, effectively blocking the production of more isoleucine via this metabolic pathway. When isoleucine levels decrease, threonine will then be able to bind to the active site of threonine deaminase, and the metabolic pathway will resume. Feedback inhibition is an important regulatory mechanism in cells to inhibit the overproduction of a product.
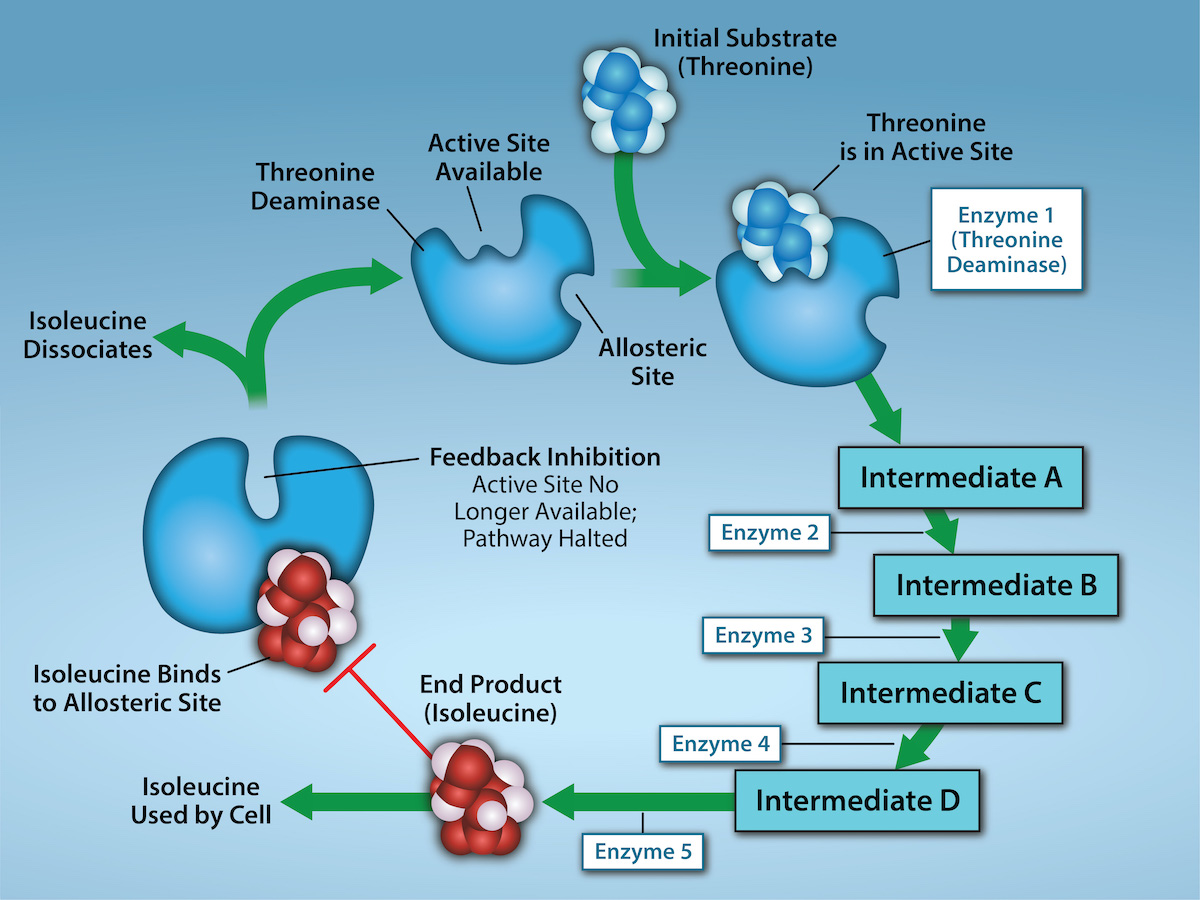
Producing both amino acids and nucleotides is controlled through feedback inhibition. Additionally, ATP is an allosteric regulator of some of the enzymes involved in the catabolic breakdown of glucose, the process that produces ATP. In this way, when ATP is abundant, the cell can prevent its further production. Remember that ATP is an unstable molecule that can spontaneously dissociate into ADP and inorganic phosphate. If too much ATP were present in a cell, much of it would go to waste. Alternatively, ADP serves as a positive allosteric regulator (an allosteric activator) for some of the same enzymes that ATP inhibits. Thus, when relative ADP levels are high compared to ATP, the cell is triggered to produce more ATP through sugar catabolism.
Link to Learning
Quick review about enzymes from FreeMedEducation:

