6.3 Energy Coupling in Metabolism: ATP and Electron Carriers
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Explain why energy coupling is necessary to drive endergonic processes forward, and how ATP often plays a role in this energy coupling.
- Explain how energy transfers via electron carriers and electrochemical gradients are involved in the energy coupling required for ATP synthesis
- Evaluate how the structure of ATP makes it an effective molecule to store chemical potential energy.
With so many chemical reactions and other energy-transfer-processes occurring in cells on a daily basis, there is a lot to keep track of. Importantly, the exergonic reactions and processes release free energy that can be used to do work, but how to cells actually make that happen? One of the big keys to cellular metabolism is small molecules that are good at storing chemical potential energy. These small molecules can “capture” the energy released by exergonic processes, and then “transfer” that energy to a place where it is needed, for example by endergonic processes. One of these small molecules is ATP (adenosine triphosphate), but we will also see in this chapter that electron carriers and electrochemical gradients are other key mechanisms for cells to effectively coordinate energy transfers during metabolism and other processes.
ATP Hydrolysis and Synthesis
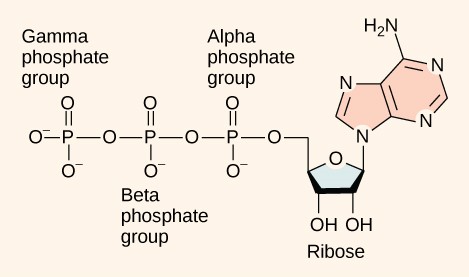
ATP is composed of an adenosine molecule bound to three phosphate groups (Figure 6.12). Adenosine is a nucleoside consisting of the nitrogenous base adenine and a five-carbon sugar, ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar are alpha, beta, and gamma. The phosphoanhydride bonds linking phosphate groups store large amounts of chemical potential energy. When broken, these bonds release large amounts free energy, which can then be used to power endergonic cellular reactions and processes.
In cellular conditions, the exergonic hydrolysis reaction of one mole of ATP has a ΔG of approximately –30.5 kJ/mol. This reaction is exergonic because the products of this reaction, adenosine diphosphate (ADP) and an inorganic phosphate group (Pi) have lower free energy than the reactants, ATP and water. ATP is relatively unstable molecule, so it must be consumed quickly in chemical reactions otherwise it will spontaneously dissociate into ADP and Pi, and the free energy will be lost as heat. Because these reactions take place using a water molecule, they are hydrolysis (“hydro” = water, “lysis” = split) reactions. ATP hydrolyzes into ADP in the following reaction:
ATP + H2O → ADP + Pi + free energy
Like most chemical reactions, the hydrolysis of ATP is reversible through the following reaction:
ADP + Pi + free energy → ATP + H2O
The synthesis of ATP requires a large input of free energy, making it a very endergonic process. Therefore cells must conduct exergonic processes to release energy for use in ATP synthesis. To do this, cells oxidize molecules like glucose, fatty acids, and amino acids via catabolic pathways to release large amounts of free energy.
Energy Coupling
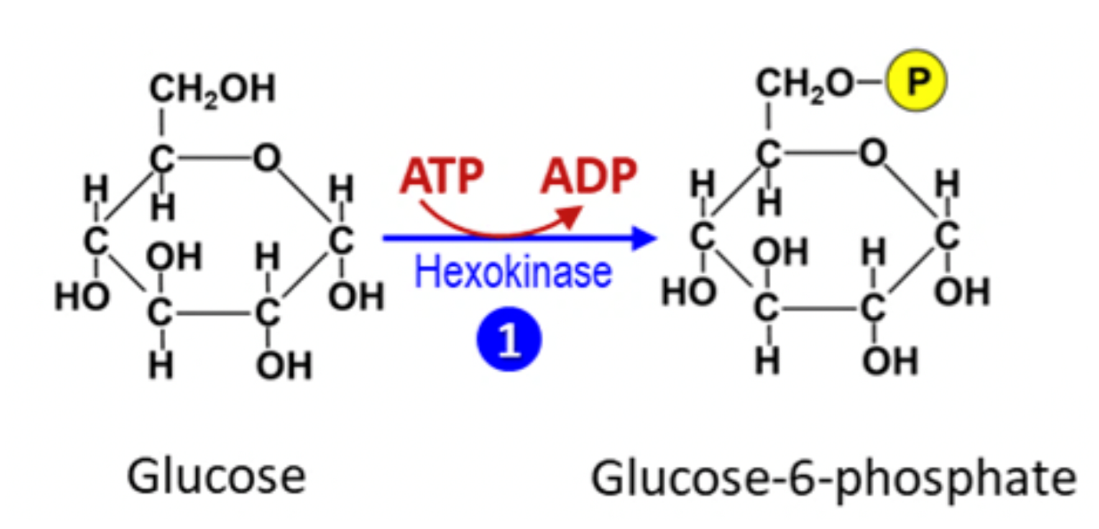
Energy coupling is the process in which free energy released by one (exergonic) reaction is used to drive another (endergonic) reaction. For example, consider the first step in the metabolic pathways of glycolysis (the beginning of cellular respiration), in which glucose is converted into glucose-6-phosphate (Figure 6.13). The phosphorylation of glucose is endergonic. Therefore, this important step in glycolysis can only proceed if it is coupled with free energy from another source. As seen in Figure 6.13, the source of this energy is ATP hydrolysis to ADP, an exergonic reaction. When we add the negative ΔG of ATP hydrolysis to the positive ΔG of glucose à glucose-6-phosphate, the net (total) ΔG is slightly negative. This allows both reactions to proceed because the free energy released by ATP is coupled to the free energy needed by the phosphorylation of glucose. Not all examples of energy coupling involve ATP, but many do. ATP is often coupled to endergonic reactions to help move them forward, which is one reason ATP is considered the energy “currency” of the cell.
Redox Reactions, Electrochemical Gradients and Energy Storage
For effective energy coupling to occur in cells, multiple chemical reactions are often needed. For example, when cells metabolize glucose and oxygen into carbon dioxide and water during cellular respiration, a large amount of free energy is released. It would be dangerous to release this free energy all at once (in a single reaction), and much of the free energy would be lost as heat rather than used to do cellular work. Consequently, cells break down glucose (and other food molecules) via many steps, involving many energy transfers. To effectively understand how cells do this, we need to talk about electrons and protons.
A redox reaction is characterized by the transfer of electrons between one molecule (or atom) and another. When cells break down glucose, glucose is being oxidized (losing electrons) and other molecules are being reduced (gaining electrons). The molecules that gain electrons increase their chemical potential energy, and this chemical potential energy can ultimately be used to power the endergonic process of ATP synthesis. In the many chemical reactions that gradually oxidize glucose during cellular respiration, the two main molecules that get reduced are NAD+ (Nicotinamide Adenine Dinucleotide) and FAD (Flavin Adenine Dinucleotide). These molecules are called electron carriers because they “carry” (move) electrons from one metabolic pathway to another, and will ultimately be oxidized (lose their electrons) to release free energy that helps power endergonic processes. In their reduced forms, they are written as NADH and FADH2 because they gain hydrogen (H) atoms when they gain electrons.
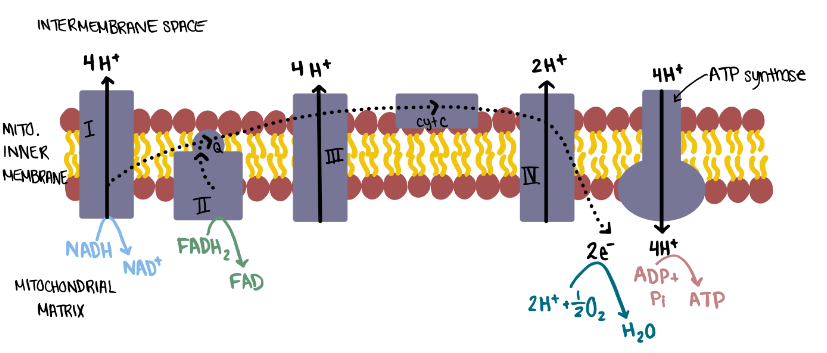
To make ATP following glucose oxidation, additional energy transformations are required that couple redox reactions to the generation of an electrochemical gradient. In mitochondria, the electrons from NADH and FADH2 are transferred to the electron transport system (ETS) in the mitochondrial inner membrane (Figure 6.14). Specifically, NADH is reduced to NAD+ at complex I of the ETS, and FADH2 is reduced to FAD at complex II of the ETS. The transferred electrons “travel” through the ETS via a series of redox reactions. These redox reactions release sufficient free energy for complexes I, III, and IV to pump protons (hydrogen ions, H+) across the mitochondrial inner membrane (Figure 6.14). This transforms chemical potential energy into another form of potential energy in a proton gradient, an electrochemical gradient that is caused by the distribution of H+ across the mitochondrial inner membrane. Recall from Chapter 5 that it is energetically favourable for ions to diffuse down electrochemical gradients. Protons in the intermembrane space diffuse across the mitochondrial inner membrane back into the mitochondrial matrix via ATP synthase, a transport protein and an enzyme. The free energy released by proton diffusion is used by cells to attach Pi to ADP, thus supporting the endergonic process of producing ATP. This elaborate process ultimately couples the release of free energy from glucose oxidation to the endergonic process of producing many ATP molecules (Figure 6.14).
Links to Learning
See an animation of the ATP-producing glycolysis process at this site.
Watch an ATP & Respiration review video from Crash Course Biology

