5.4 Active Transmembrane Transport
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Compare and contrast the mechanisms of primary and secondary active transport, including the types of carrier proteins and energy sources involved.
- Explain how primary active transport supports secondary active transport.
- Give examples of carrier proteins involved in both types of active transport.
In active transmembrane transport, solutes move across a membrane up (against) their concentration gradient (neutral solutes) or electrochemical gradient (charged solutes). This requires a carrier protein and an energy source to move solutes away from equilibrium. In other words, for a substance to move across a membrane against its concentration or electrochemical gradient, the cell must expend energy. Active transport is used for a variety of cellular processes, such as nutrient uptake (e.g., sugars, amino acids), waste/water removal, and maintaining non-equilibrium concentrations of specific ions. Active transport cannot be used for solutes that can cross membranes via simple diffusion. We will two types of active transport in this section: primary active transport and secondary active transport.
Thermodynamics of Moving Against a Gradient
One useful framework for thinking about active transmembrane transport is a thermodynamic framework. This will only really make sense if you read ahead: peruse Chapter 6.1 and then come back here. (Or ignore skip this section for now and come back to it after you’ve read Chapter 6.1 later in the course). When solutes move down (with) their concentration gradient or electrochemical gradient (as in passive transport), that movement is an endergonic process (negative free energy; DG < 0), and should happen spontaneously without any energy input from the cell. However, when solutes move up (against) their concentration gradient or electrochemical gradient (as in active transport), that movement is an endergonic process (positive free energy; DG > 0). The cell must therefore pair this endergonic process with an exergonic process to make the net (total) free energy of transport slightly negative. This exergonic process is the “energy source” that is often described for active transport.
Two energy sources exist for transporting low-molecular weight solutes across membranes via active transport. Primary active transport directly uses energy from an exergonic chemical reaction (usually ATP hydrolysis) to move solutes against a concentration or electrochemical gradient across a membrane. One example of this is the active transport of hydrogen ions (protons, H+) via the proton pump in Figure 5.26; you can see the ATP being hydrolyzed to ADP + Pi by the pump, a chemical reaction that releases enough free energy to move H+ against its electrochemical gradient. This proton pump is an example of a uniporter carrier protein (Chapter 5.2). Secondary active transport also requires an energy source, but does not directly receive that energy from an exergonic chemical reaction (like ATP hydrolysis). Instead, secondary active transport uses energy from an electrochemical gradient of a different molecule. This other gradient is a form of potential energy (Chapter 6.1) and is usually established through primary active transport. In the example in Figure 5.26, a H+ gradient has been set up by the proton pump via primary active transport. The sucrose-H+ cotransporter then uses secondary active transport to move sucrose up its concentration gradient. H+ diffuses (down its gradient) through the same transmembrane transport protein as sucrose, releasing free energy that can power the active transport of sucrose. The sucrose-H+ co-transporter is an example of a symporter carrier protein (Chapter 5.2).
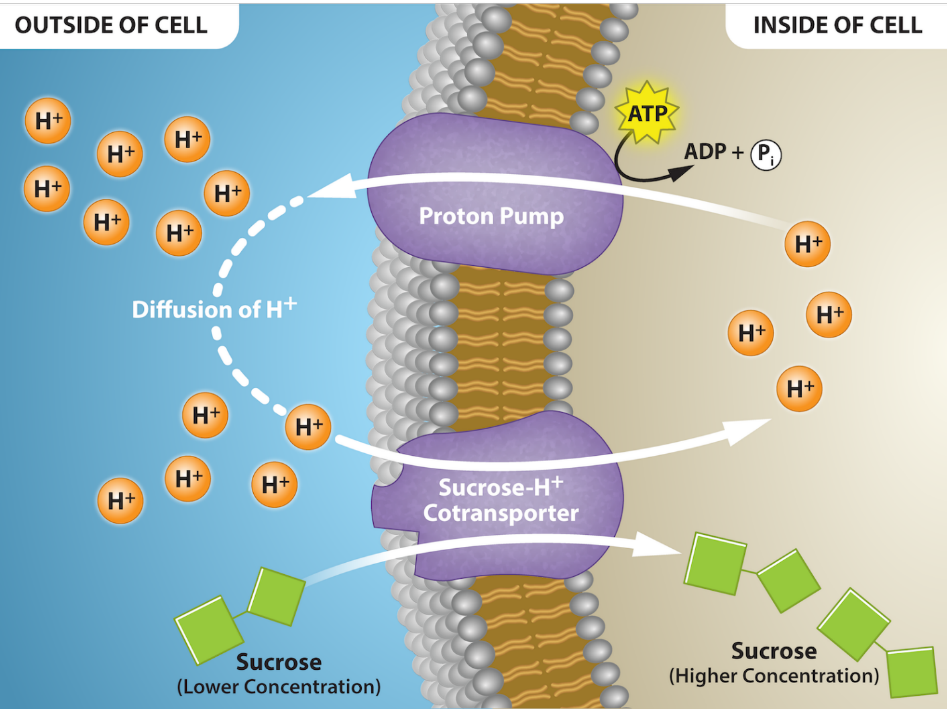
Primary Active Transport
As described above, primary active transport directly uses energy from a chemical reaction (usually ATP hydrolysis) to move solutes against a concentration or electrochemical gradient across a membrane. The proteins involved in primary active transport are usually both carrier proteins (for solute transport) and enzymes (for ATP hydrolysis). The proton pump in Figure 5.26 is one example: it is both a uniporter carrier protein that transports protons (H+) and an enzyme that catalyzes the hydrolysis of ATP. Another classic example is the sodium-potassium ion pump, also called sodium-potassium ATPase (Na+/K+ ATPase). Figure 5.27 shows the changes in shape of this antiporter carrier protein that moves Na+ and K+ across a membrane, and the catalytic (enzyme) region of the protein that facilitates ATP hydrolysis.
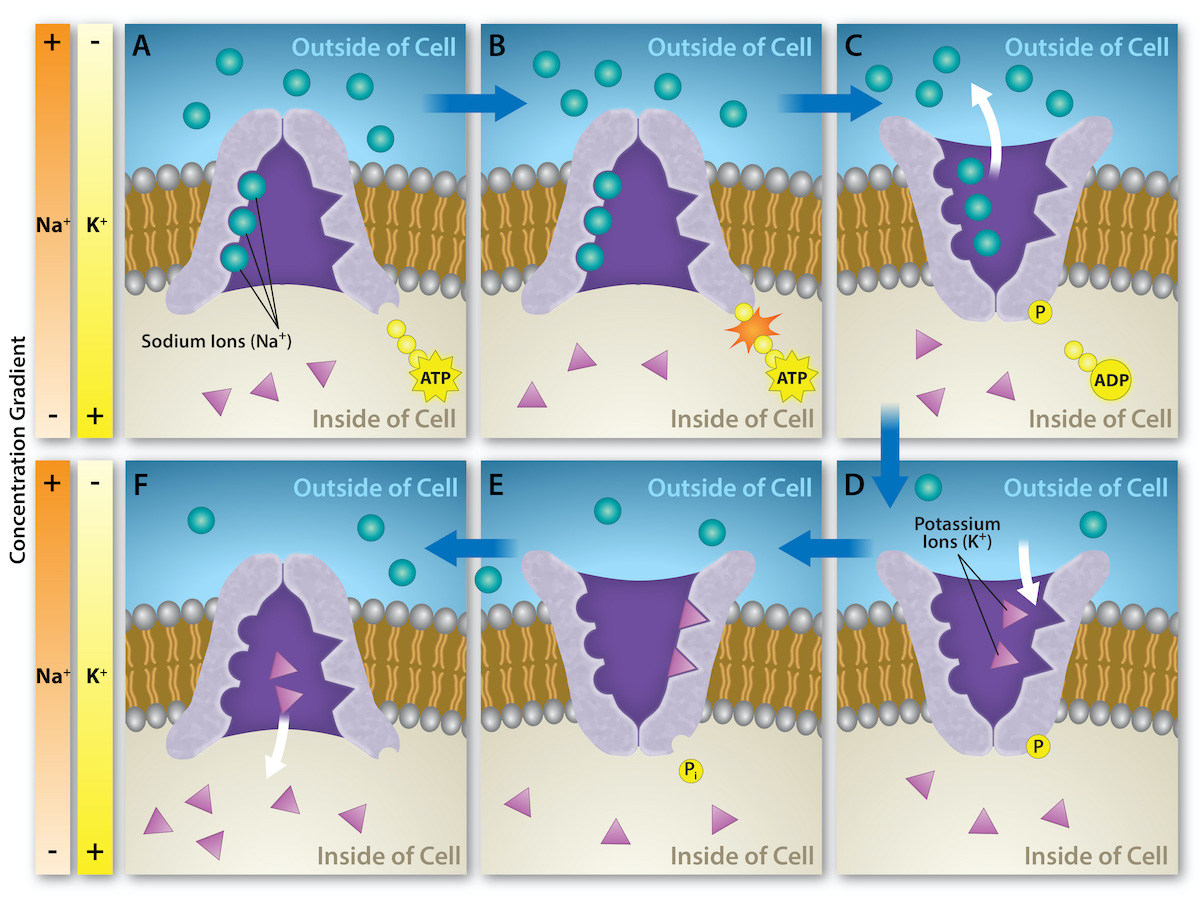
Link to Learning
Examples of Carrier Proteins in Primary Active Transport
There are four major types of transport ATPase proteins that link primary active transport to ATP hydrolysis. They are all carrier proteins with catalytic regions, but they differ in their locations and functions. These proteins are also often called “pumps” because they are involved in active transport.
P-type ATPases (“P” for plasma membrane) are found in bacterial and eukaryotic membranes. They use the energy derived from ATP hydrolysis to pump ions across the membrane, including: Na+, K+, Ca2+ and some heavy metals. These ATPases play crucial roles in the ionic balances that regulate signal transduction, pH, metabolism, membrane potential and homeostasis. The proton pump in Figure 2.26 and sodium-potassium pump (Figure 2.27)are both examples of a P-type ATPases.
V-type ATPases (“V” for vacuole) are mostly found in the vacuoles of eukaryotes. They use energy from ATP hydrolysis to pump hydrogen ions (H+, protons) from the cytoplasm to the lumen of the organelle, creating a proton gradient. These proton pumps are found in the membranes of intracellular organelles, such as vacuoles (including the central vacuole of plant cells), vesicles, lysosomes, and the Golgi apparatus. These ATPases are involved in several cellular processes, notably endocytosis/exocytosis, and pH maintenance.
F-type ATPases (“F” for factor) are found in the inner membranes of mitochondria, chloroplast thylakoid membranes, and bacterial plasma membranes. These ATPases use the energy derived from ATP hydrolysis to generate a proton (H+) gradient across the membrane. They can also run in reverse: protons can diffuse (down a gradient) through F-type ATPases, releasing energy that can be used to synthesized ATP (reverse of ATP hydrolysis; see Chapter 6.3). F-type ATPases are vital element in cellular metabolism and their malfunction can lead to a variety of disorders, such as neurodegenerative disorders or mitochondrial myopathies.
ABC-type ATPases (“ABC” for “ATP Binding Cassette) comprise a large family of membrane-bound transport proteins, in both eukaryotes and prokaryotes, that use the energy derived from ATP hydrolysis to move a variety of substrates across the membrane. The term “cassette” refers to the catalytic domain that binds ATP and hydrolyzes it during the transport process to provide energy for solute movement. This larger group of ATPases include subcategories of importers and exporters. In prokaryotes, importers are involved in the uptake of nutrients including vitamins, macromolecules, and salts. Exporters are involved in the transport of waste and toxic products. These ATPases can also act as multidrug resistance transporters that removes drugs and antibiotics from the cell.
Secondary Active Transport
Secondary active transport involves the simultaneous transport of two or more types of solutes; one moving favourably down (with) its gradient, driving the movement of the other solute up (against) its gradient. Specific carrier proteins are required to facilitate the movement of these two solutes, and these carriers can be either symporters or antiporters, but not uniporters (Figure 5.17). Although they are involved in active transport, these carrier proteins do not directly require ATP to work. Usually, secondary active transport requires an electrochemical gradients (usually created by primary active transport) that it can use as an energy source.
For example, consider a mechanism of secondary active transport than can be used to move glucose up its concentration gradient (Figure 5.28) into an animal cell. The primary active transport by Na+/K+ ATPase generates an electrochemical gradient involving sodium. A glucose-Na+ symporter is a carrier protein that allows Na+ to diffuse down its electrochemical gradient, releasing energy that can be used to move glucose up it’s concentration gradient through the same transport protein (Figure 5.28). Many nutrients, such as amino acids and glucose, enter animal gut cells this way. While animals rely on sodium ions to drive secondary active transport, most other organisms rely on a proton (H+, hydrogen ion) gradient. Plants and fungi use an ATP-driven proton pump to maintain the proton electrochemical potential and uptake organic substances through proton symport (e.g., Figure 5.26).
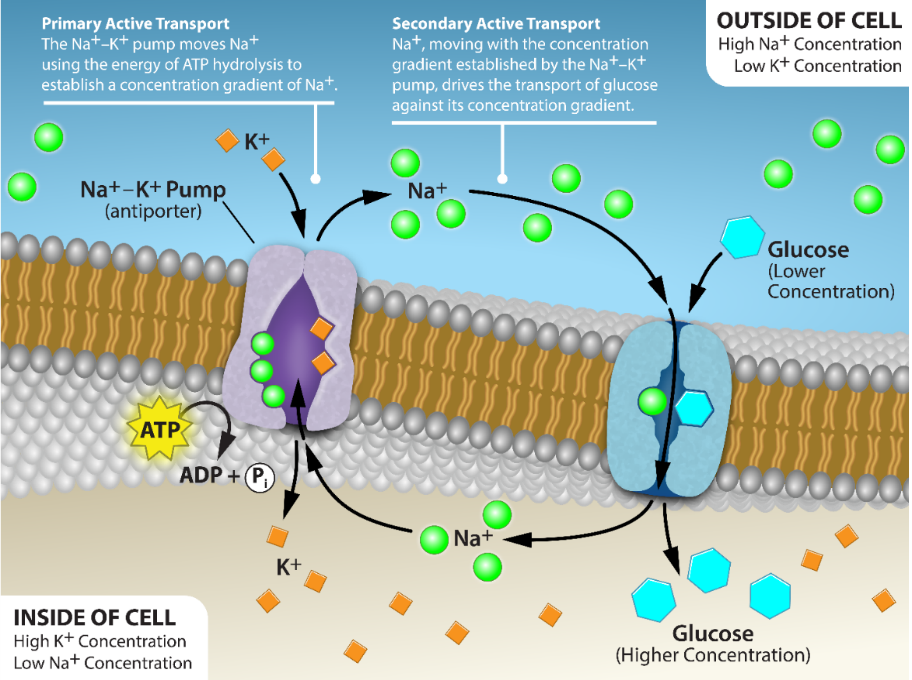
Link to Learning

