5.1 Membrane Components and Structure
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Recognize the structure and describe the function of lipids, proteins, and carbohydrates in cellular membranes.
- Understand the importance of maintaining cellular membranes in a liquid crystalline (fluid) state.
- Explain why and how temperature alters membrane fluidity.
- Evaluate the contribution of phospholipid fatty acid tails, phospholipid head groups, and cholesterol to membrane fluidity.
Membrane Components
The fluid mosaic model describes the overall structure and function of the cell membrane as two fluid layers of phospholipids, with proteins in and on each layer (Figure 5.2). The membrane is described as “fluid” because both proteins and phospholipids can easily move laterally through the membrane. The “mosaic” part of this model refers to the presence of many proteins, lipids, and carbohydrates scattered throughout the lipid bilayer, reminiscent of mosaic tile art pieces (Figure 5.1), which contain many different components. Proteins and lipids are the main components of the membrane, however the protein to lipid ratio varies among different types of cells and organelles. For example, human erythrocyte (red blood cell) plasma membranes have a low protein to lipid ratio, whereas chloroplast membranes have a higher protein/lipid ratio.
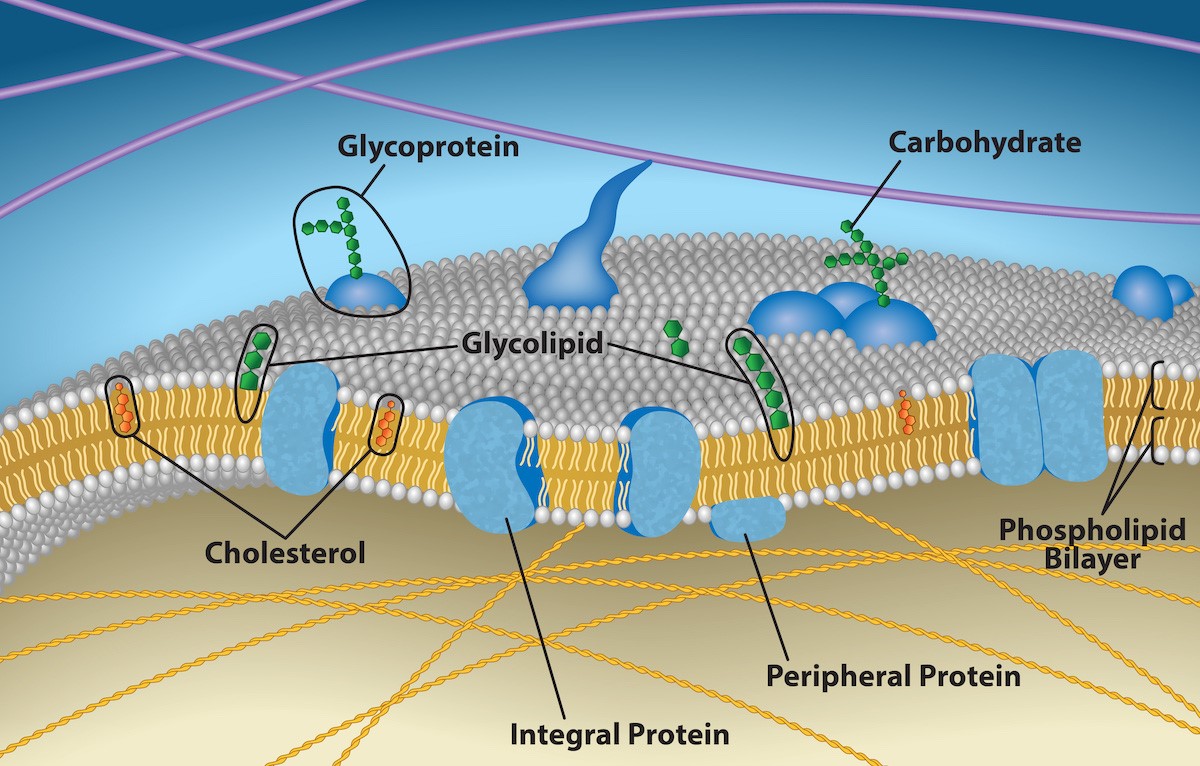
The first part of this chapter will consider the structure and function of membrane components. First, let’s give a brief overview of the components in Figure 5.2. Phospholipids arrange in two layers, with their hydrophobic tails pointing towards the inside of the bilayer, and their hydrophilic heads pointing towards the exterior of the bilayer. Sterols (e.g. cholesterol) are additional lipids that can be found in each layer. Integral proteins are embedded in the membrane, peripheral proteins are found on the perimeter of the inner or outer face of the membrane, and lipid-anchored proteins are covalently attached to lipids in the membrane. Glycolipids and glycoproteins are carbohydrates chains attached to lipids and proteins, found in the outer layer of the membrane. Now, let’s dive into some details!
Lipids
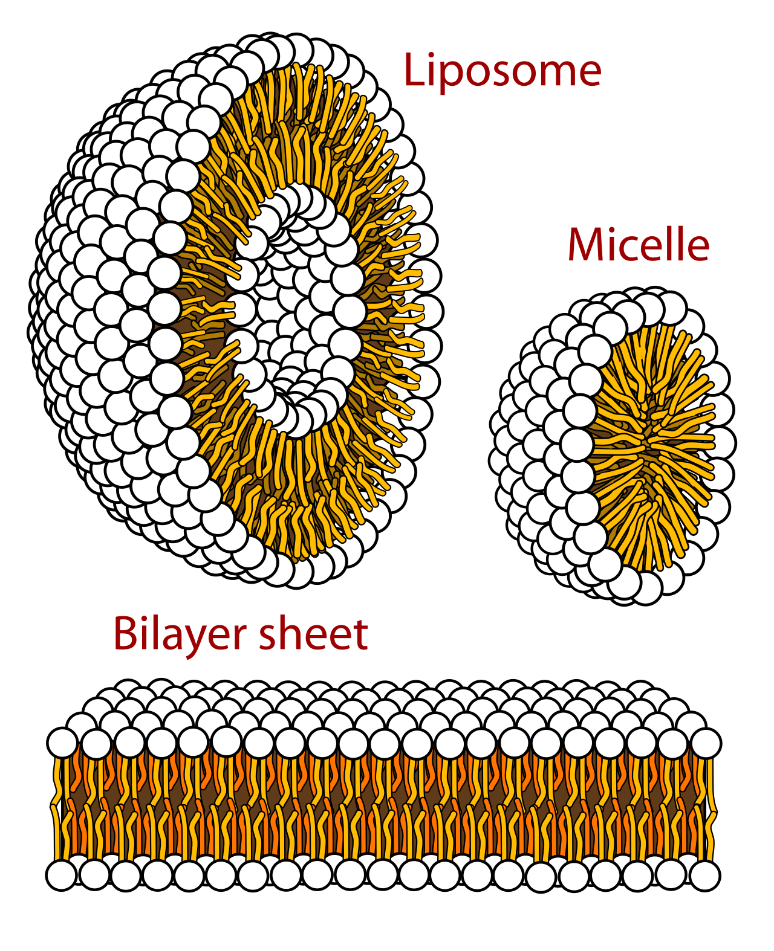
Cellular membranes can contain three types of lipids: phospholipids, sphingolipids and sterols. All of these types of lipids are predominantly hydrophobic, but also contain hydrophilic regions, which is important for the overall structure of membranes. Molecules with hydrophobic and hydrophilic portions are known as amphipathic or amphiphilic. This amphipathic property causes the hydrophobic portions of lipids to face each other, and the hydrophilic portions to face outward to interact with an aqueous (water-based) environment, an arrangement that is energetically favourable. This allows amphipathic lipids like phospholipids to form a variety of structures in solution, like spheres, bilayer spheres, or bilayer sheets (Figure 5.3). The cell membrane can be thought of as a lipid-bilayer sphere.
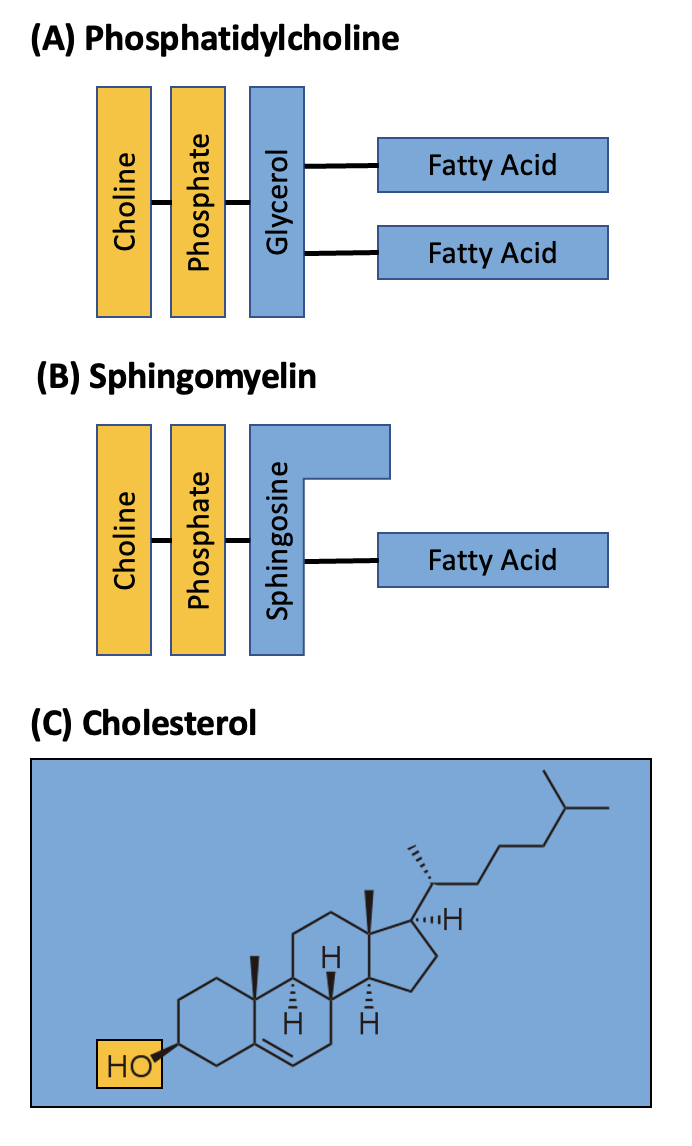
Phospholipids consist of a three-carbon glycerol backbone with two fatty acid molecules attached to carbons 1 and 2, and a phosphate-containing group attached to the third carbon; you can refer back to Chapter 3.3 for a reminder of the full chemical structure. In cellular membranes, the phosphate is usually linked to an additional organic molecule to the glycerol backbone, for example, choline (Figure 5.4). The glycerol and phosphate-containing group form a hydrophilic, negatively charged “head” region, which can form hydrogen bonds with water and other molecules on either side of the membrane. The two fatty acids form hydrophobic, neutral “tails”, which cannot form hydrogen bonds with water in the environment.
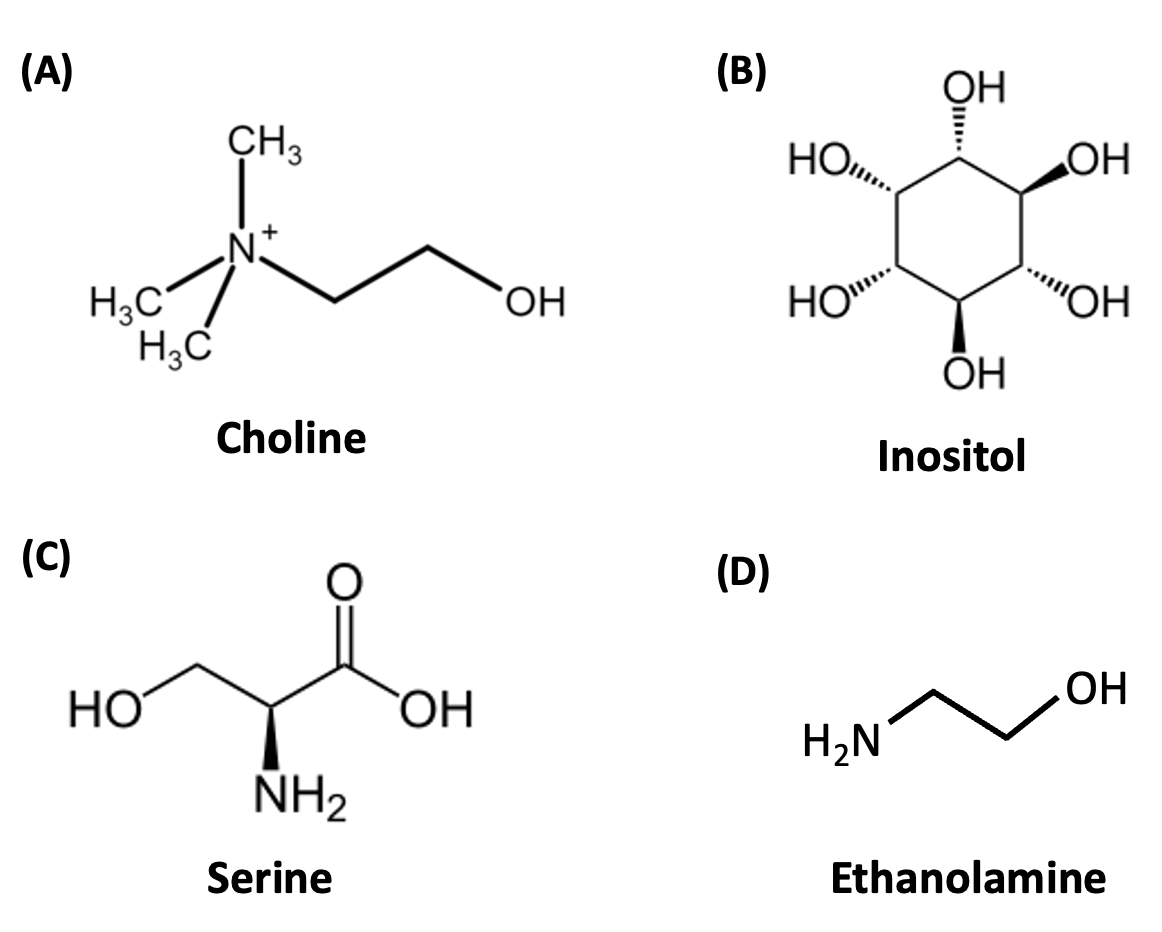
A phospholipid’s hydrophilic head can vary in size, shape and charge, depending on the group attached to the phosphate. A few common groups are choline, inositol, serine and ethanolamine (Figure 5.5). These form phosphatidylcholine, phosphatidylinositol, phosphatidylserine, and phosphatidylethanolamine, respectively. Figure 5.4 shows the position of the choline group on phosphatidylcholine. The inositol, serine and ethanolamine groups on other phospholipids would be found in the same region as the choline group.
Sphingolipids are a type of phospholipid that have a hydrophobic tail made up of only one fatty acid chain and a sphingosine molecule (Figure 5.4). Sphingosine is derived from the amino acid serine and a long hydrocarbon chain; this hydrocarbon chain runs parallel to the fatty acid chain in a sphingolipid. Like phospholipids, the hydrophilic head group on sphingolipids can vary. An example of the chemical structure of a sphingolipid can be viewed in the carbohydrates section below when discussing glycolipids.
Sterols are another type of lipid found in the cell membrane. They are multi-ringed structures that are mostly hydrophobic, except for a hydrophilic hydroxyl group. In the membrane their hydrophobic regions associate with phospholipid tails, and their hydrophilic regions associate with phospholipid head groups. Cholesterol is a common sterol found between phospholipids in the animal cell membranes, with a small hydrophilic portion (hydroxyl group, OH) (Figure 5.4).
Proteins
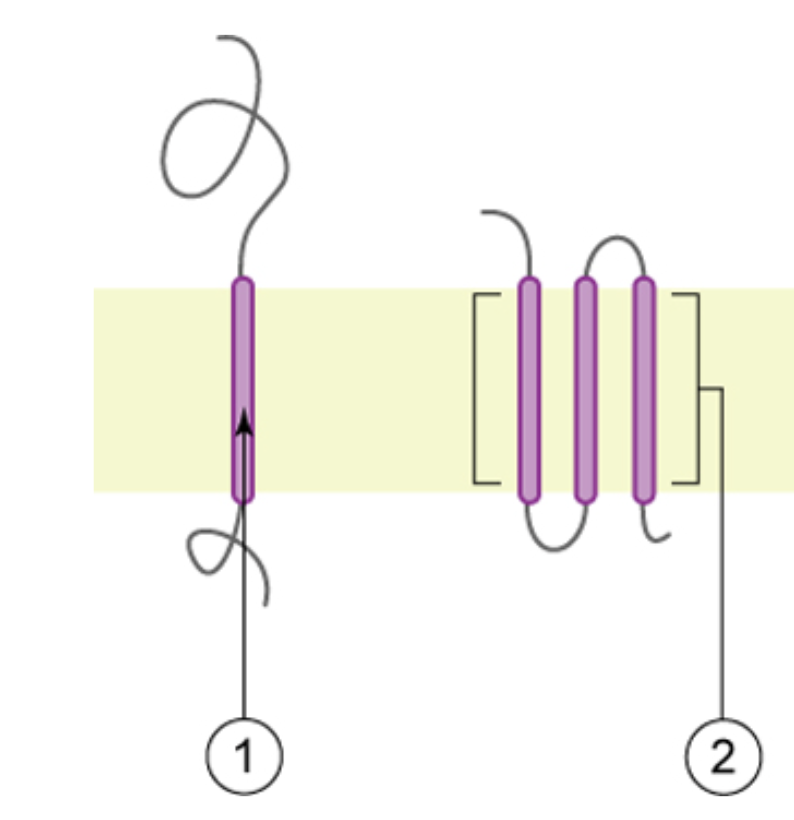
Proteins can have many functions in membranes, including (but not limited to) serving as transport channels, receptor proteins, enzymes, and structural attachments for cytoskeleton fibers. If you need a refresher on general protein structure, please refer to Chapter 3.4. Membrane proteins can be classified integral, peripheral, or lipid-anchored based on how they interact with hydrophobic and hydrophilic regions of a lipid bilayer (Figure 5.6). These proteins have a range of functions, including transmembrane transport and cell signalling, which will be discussed later in this chapter. They can also be important components of cell-cell connections (see Chapter 4.6).
Integral proteins, as their name suggests, integrate completely into membranes due to their hydrophobic and hydrophilic regions. Their hydrophobic segments are adjacent to phospholipid tails, and their hydrophilic segments are adjacent to phospholipid heads and protrude into the fluid adjacent to the membrane (e.g., into the cytosol) (Figure 5.6). Integral monotopic proteins span only part of the membrane, associating with a single layer of phospholipids. Other integral membrane proteins, known as transmembrane proteins, span across both layers of the membrane. A transmembrane protein segment usually consists of 20-30 amino acid residues which have hydrophobic R-groups and are arranged in an alpha helix. Single–pass integral membrane proteins have only one transmembrane segment, and therefore span the bilayer once. Conversely, multi–pass proteins can have anywhere from 2 to 20 segments spanning the membrane (Figure 5.6). Multi–subunit integral membrane proteins also span across the bilayer several times, however these more complex proteins are made of two or more polypeptides.
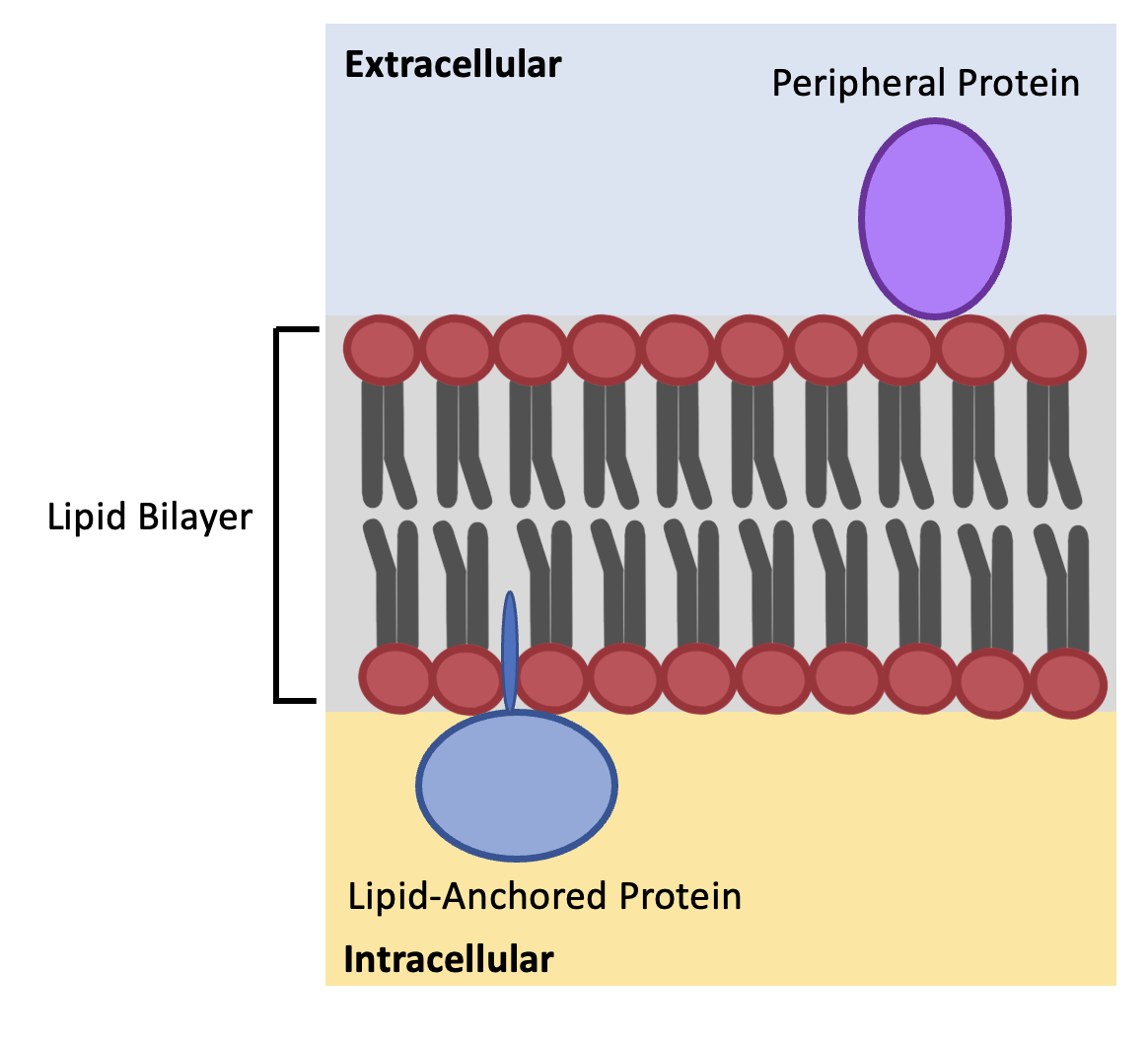
Peripheral membrane proteins lack hydrophobic amino acid residues on the surface of the protein; therefore, they cannot penetrate the lipid bilayer. These proteins attach – via hydrogen bonds and weak electrostatic interactions – to hydrophilic phospholipid heads and hydrophilic regions of integral membrane proteins (Figure 5.7)
Lipid-anchored membrane proteins also lack hydrophobic regions on their surface, so they attach to the membrane via covalent bonds to lipids which are embedded in the membrane. In eukaryotic cells lipid-anchored proteins on the inner surface of a plasma membrane are attached by a fatty acid chain or an isoprenyl group (Figure 5.7). Furthermore, lipid-anchored proteins on the outer surface of a plasma membrane are attached to a glycolipid found in the outer monolayer, called glycosylphosphatidylinositol (GPI).
Carbohydrates
Carbohydrates do not penetrate the lipid bilayer because they are hydrophilic; however, they are still an important part of the cell membrane. For a refresher on general carbohydrate structure, consult Chapter 3.2. Carbohydrates can be found on the exterior surface of the plasma membrane and are bound either to proteins (glycoproteins) or to lipids (glycolipids) (Figure 5.2). Carbohydrates from glycoproteins and glycolipids on the outer surface of an animal cell membrane, can be referred to as the glycocalyx (“sugar coating”). The glycocalyx has specific and unique patterns, to allow the immune system to differentiate between body cells (“self”) and foreign cells or tissues (“non-self”). The glycocalyx is also highly hydrophilic and attracts large amounts of water to the cell’s surface. This improves the cell’s ability to obtain substances dissolved in the water. The glycocalyx is also important for cell to cell attachment, among many other things.
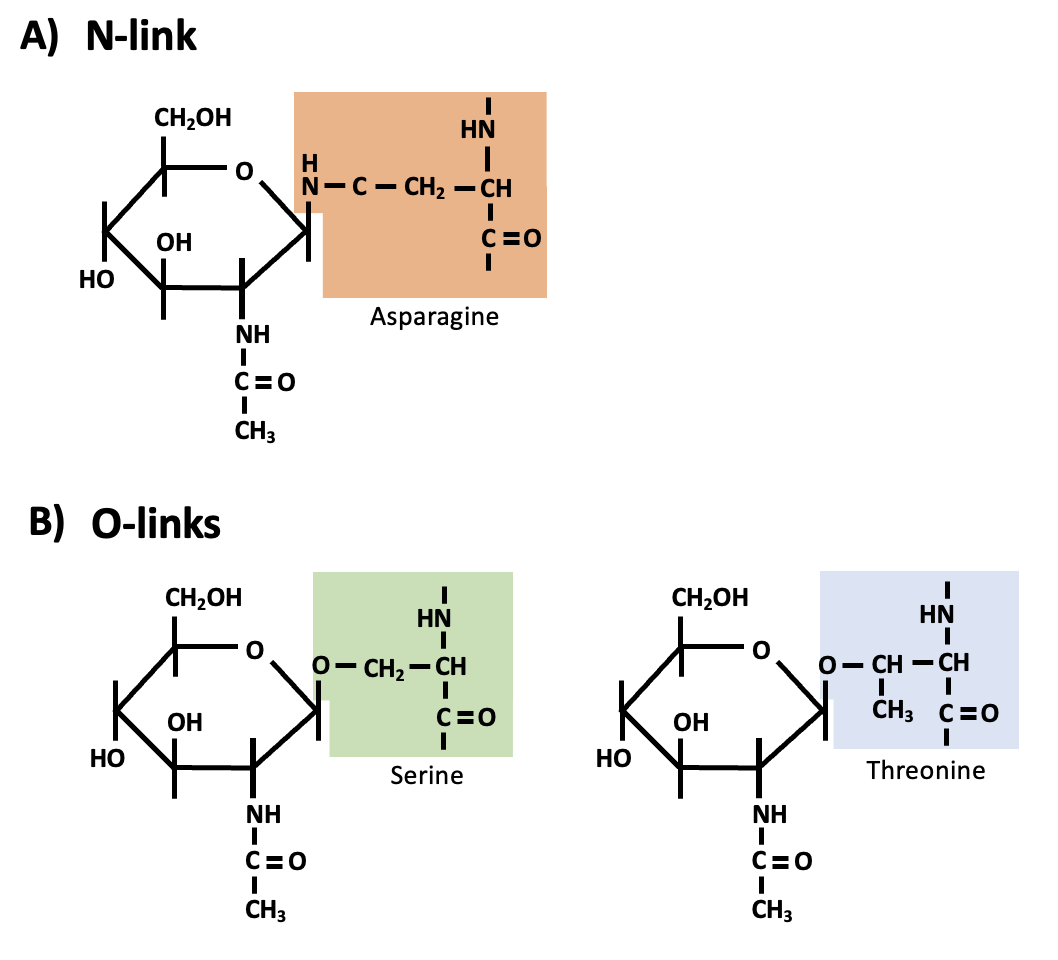
Glycoproteins are oligosaccharides (short carbohydrate chains) attached to proteins by a N-link or an O-link (Figure 5.8). N-linked oligosaccharides attach to the nitrogen atom of an amino group in the side chain of an amino acid residue. O-linked oligosaccharides attach to the oxygen atom of a hydroxyl group in the side chain of an amino acid residue. In animal cells, glycoproteins on the cell surface are important for cell-cell recognition (e.g., for your immune system to recognize which cells are yours, and which cells are potential pathogens).
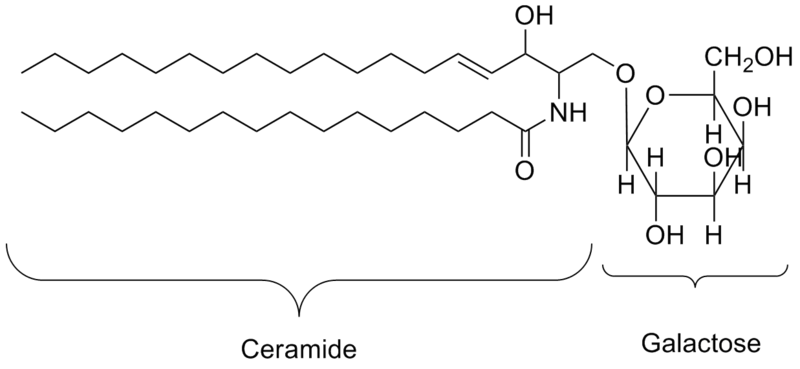
Glycolipids consist of a carbohydrate group attached to a phospholipid or a sphingolipid, forming a glycoglycerolipid or a glycosphingolipid, respectively. Cerebrosides and gangliosides are two common glycosphingolipids in animals. Cerebrosides have a neutral monosaccharide as their head group (Figure 5.9) and are an important component in the cell membranes of animal neuron and muscle cells. Gangliosides are also important in animal neuron cell membranes, and have an oligosaccharide with a net negative charge as their head group.
Membrane Fluidity
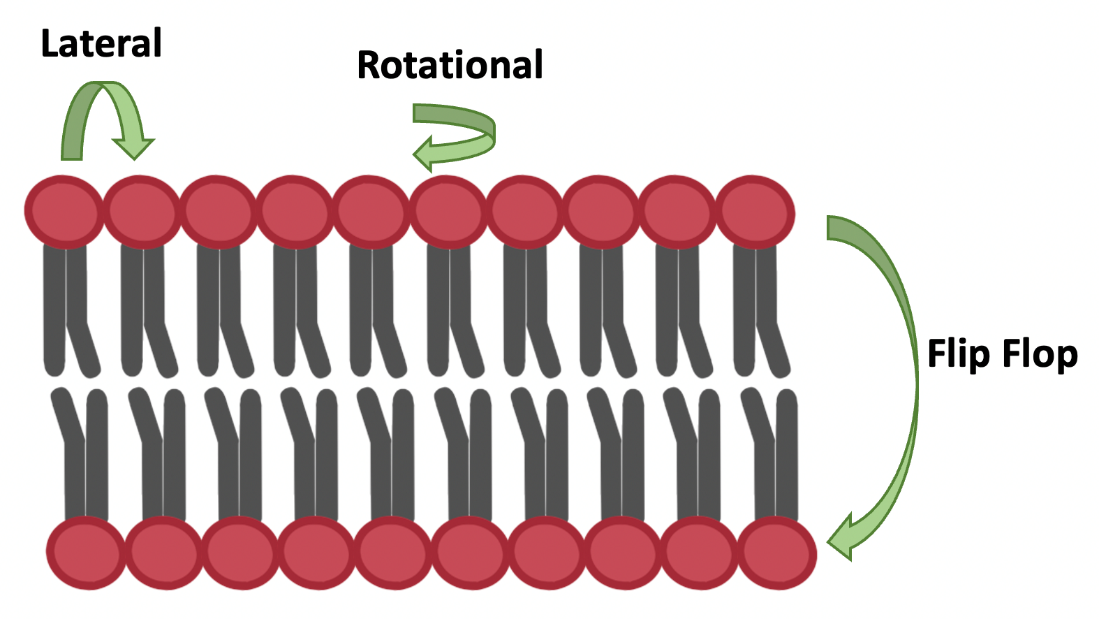
Membrane fluidity can be defined as the extent of molecular disorder and molecular motion within a lipid bilayer. As described by the fluid mosaic model, lipids, proteins and carbohydrates all move around the cell membrane. Some common movements of phospholipids in the membrane include lateral movement, rotational movement and “flip-flopping” (Figure 5.10). Fluidity is important for membrane function, because it is important for the membrane to be somewhat flexible (e.g., to support vesicular transport; Chapter 5.5) and avoid becoming solid (because solid membranes lose all function and structural integrity). Membrane fluidity is affected by the types of phospholipids, the temperature, and the presence of cholesterol. When phospholipid tails are tightly packed together the membrane has low fluidity, and when they are not packed tightly the membrane has high fluidity.
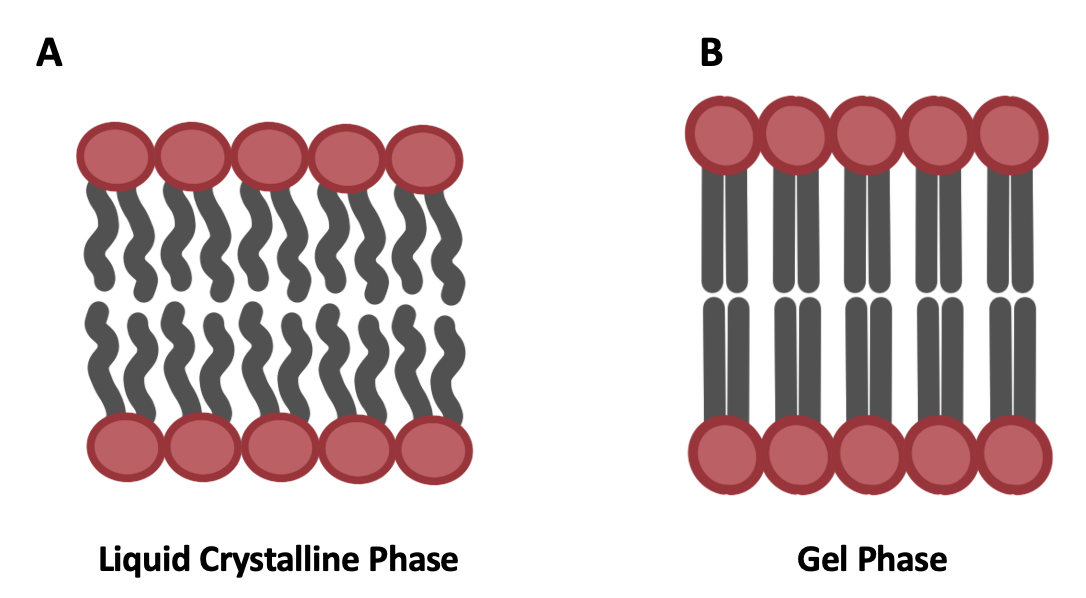
All membranes have a temperature of the membrane phase transition (Tm), between liquid crystalline (fluid) and gel phases. In a normal functioning membrane T > Tm (warmer), and the membrane is in the liquid crystalline phase with phospholipids moving freely (Figure 5.11A). At very high temperatures, membranes can become too fluid and start to dissociate. When T < Tm (cooler), the membrane transitions to the gel phase and phospholipids become tightly packed, so membrane fluidity is decreased (Figure 5.11B). As an analogy, think about spreading butter on bread. Butter is easiest to spread when it is a little bit soft (analogous to the liquid crystalline phase). You don’t want that butter to be fully liquid (e.g., what happens if you heat the butter up too much) not too solid (e.g., right out of the fridge). You want that butter to be somewhere in between totally liquid and totally solid for optimal sandwich preparation, and the same is true for membranes! (Minus the sandwich bit.) Cells can adjust the types of lipids that are in their membranes to keep fluidity at an optimal level, for example when temperature increases or decreases.
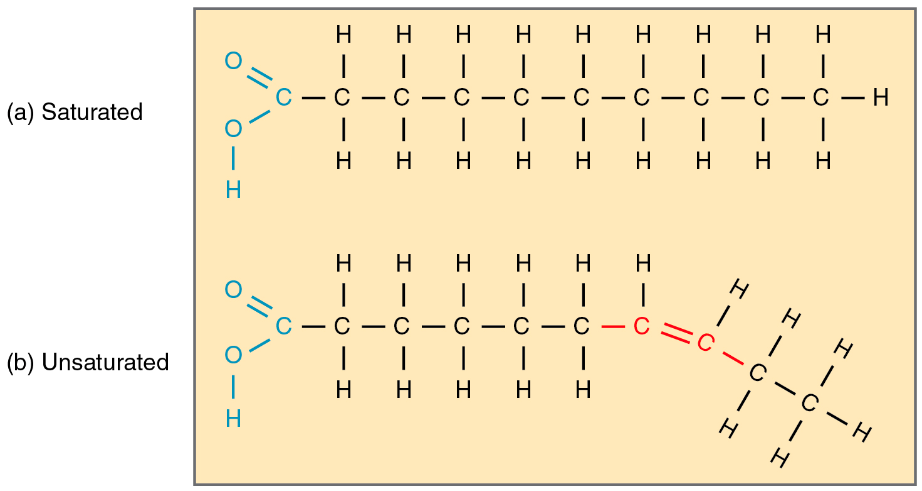
The fatty acid tails of phospholipids can vary in saturation and in length, both factors which affect membrane fluidity. Recall that fatty acids are long hydrocarbon chains which can either be saturated or unsaturated (Chapter 3.3). Saturated fatty acids (Figure 5.12A) are those which do not contain any carbon-carbon double bonds, therefore, they have the maximum number of hydrogens per carbon. Unsaturated fatty acids (Figure 5.12B) are those which contain one or more carbon-carbon double bonds, so they do not have the maximum number of hydrogens per carbon. The double bond in unsaturated fatty acids causes a bend in the chain which prevents fatty acid tails from tightly packing. Therefore, unsaturated fatty acids in phospholipids increase membrane fluidity, and saturated fatty acids reduce membrane fluidity. Long fatty acids have more area available for intermolecular interactions to form with other fatty acids, therefore they decrease membrane fluidity. Short fatty acids have less area available for intermolecular interactions to form, therefore they increase membrane fluidity.
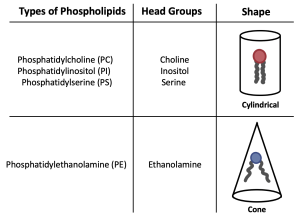
The hydrophilic head of phospholipids can also affect membrane fluidity. The head group can cause the phospholipid fatty acid tails to assume a cone or a cylinder conformation. For example, phosphatidylcholine (PC), phosphatidylinositol (PI) and phosphatidylserine (PS) form a cylinder shape, whereas phosphatidylethanolamine (PE) forms a cone shape (Figure 5.13). The cylinder shape allows fatty acid tails to pack more tightly together, therefore decreasing membrane fluidity. The cone shape puts more space between phospholipids, therefore it increases membrane fluidity.
Cholesterol affects the membrane fluidity of animal cell membranes differently at high and low temperatures. Recall, cholesterol is a series of hydrocarbon rings, making it a very rigid and bulky molecule (Figure 5.4). At high temperatures, cholesterol can help reduce membrane fluidity due to its rigid (not fluid) structure. At low temperatures, cholesterol’s rigid structure can help increase membrane fluidity because it physically prevents phospholipids from tightly packing too close together.

