4.5 The Cytoskeleton
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Recognize the structure (including size, polarity) and describe the functions of microfilaments, intermediate filaments, and microtubules
- Evaluate the role of ATP and GTP in cytoskeleton polymerization and depolymerization
- Explain the how motor proteins interact with different components of the cytoskeleton to promote movement (of cells, within cells)
- Compare and contrast the structure and function of cilia and flagella
- Give examples of methods that can be used to visualize microfilaments, intermediate filaments, and microtubules with microscopy
- Give examples of how the abundance or structure of each cytoskeletal component varies among different cell types
As briefly discussed in Chapter 4.2, the cytoskeleton is a protein framework that has multiple roles, including structural support, locomotion, cell division, and bulk transport. The cytoskeleton is composed of three main components: microfilaments, intermediate filaments, and microtubules. Each component is fibrous (long and “stringy”) and made up of specific proteins. Throughout this chapter section we will discuss the structure and functions of these cytoskeletal components in more detail. We will also look at a two specialized cellular structures that utilize microtubules for cellular locomotion: flagella and cilia.
Microfilaments
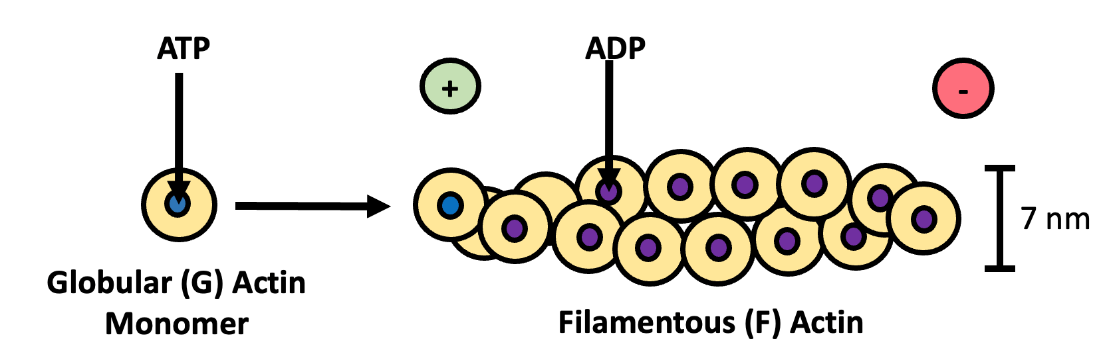
Of the three types of protein fibers in the cytoskeleton, microfilaments are the narrowest. They are 7 nm in diameter and between 100 nm and 2 µm in length. Microfilaments are composed of multiple copies of a protein called actin. When actin is first synthesized, it is a globular protein, and thus referred to as G-actin. Multiple G-actin subunits bind to each other, creating a filamentous form of actin known as F-actin (Figure 4.17). ATP is required for G-actin to polymerize (assemble) into F-actin. Two strands of F-actin then coil around each other creating the long and narrow microfilament. The microfilaments have a plus (+) and a minus (-) end to denote some polarity to the model (i.e. the two free ends are different), but the ends of the filaments are not electrically charged.
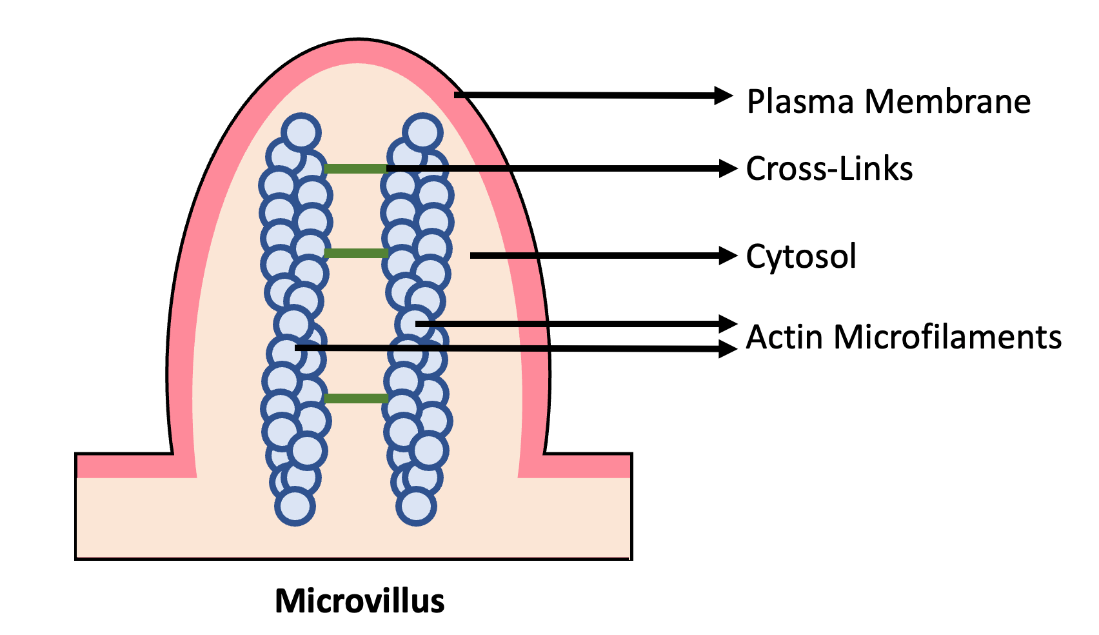
Many microfilaments can come together to form a mesh framework. This formation gives them strong tensile force, which is imperative for their function. The mesh framework is particularly abundant underneath the cell membrane. Due to its tensile strength, it can support the shape of the cell membrane, especially in cells that do not have a cell wall. Because actin is so thin, it can support very delicate shapes such as the microvilli (cell membrane folds) of cells lining animal (including human) intestines (Figure 4.18).
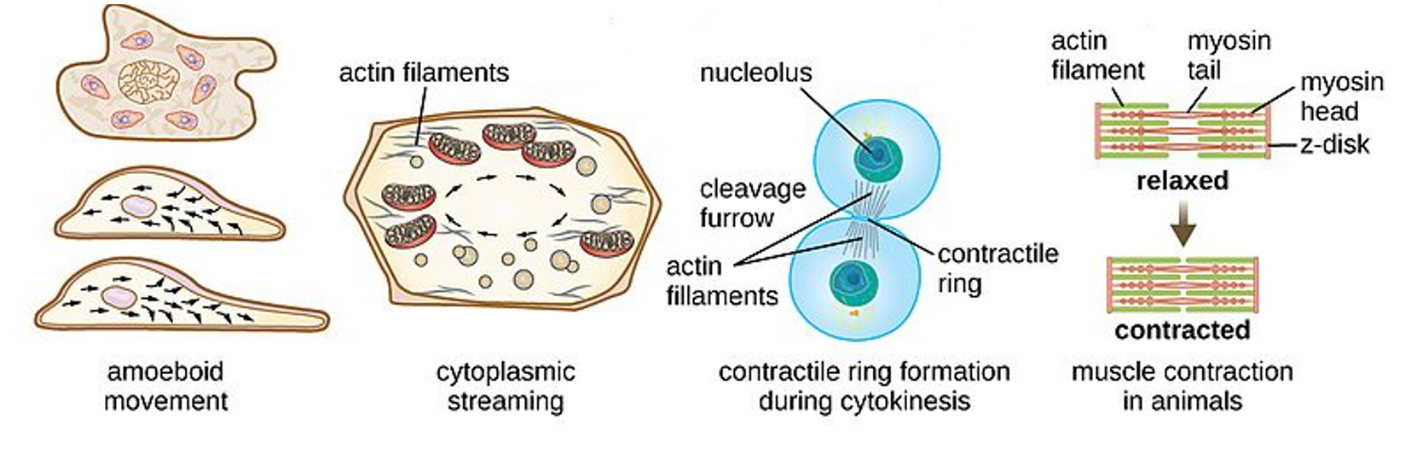
Microfilaments can quickly depolymerize (disassemble) and repolymerize (reassemble), allowing them to “move”. This is done by adding or removing G-actin monomers, which are loose in the cytosol, to the + or – end of the filament, through ATP hydrolysis. Whenever, G-actin is abundant, it is more likely to be added to the + end and be removed from the – end. When both processes occur at the same rate, the total length of the actin filament remains the same, but it “moves” in one direction. This is referred to as “treadmilling.” If this actin treadmilling occurs under the plasma membrane, this can enable cells to quickly change shape and move. For example, changes in the actin cytoskeleton under the cell membrane allow some animal cells and protists (without a cell wall) to move using amoeboid motion (Figure 4.19). White blood cells, which are responsible for fighting infections, use this type of actin-driven motion to move to an infection site where they can phagocytize pathogens.
Actin microfilaments can also facilitate movement by interacting with myosin, a motor protein. These movements are important in animal cell division, cytoplasmic streaming, muscle cell contraction, and phagocytosis. Myosin converts chemical energy from ATP hydrolysis into mechanical energy, which pulls the actin filaments along. Actin and myosin are abundant in muscle cells and are responsible for your muscle contractions. In addition, dividing eukaryotic cells also utilize this interaction create a contractile ring that facilitates division of one cell into two during cytokinesis of mitosis and meiosis. Cytoplasmic streaming, which is most prevalent in plant cells, is the actin-driven movement of the cytoplasm to transport nutrients, proteins, and organelles throughout the cell. The interactions between actin and myosin also help with amoeboid motion described in the previous paragraph. See Figure 4.19 for a depiction of each actin-driven movement.
Link to Learning
Intermediate Filaments
Intermediate filaments are thus named because their diameter, 8 to 10 nm, which is between those of microfilaments and microtubules. The basic unit of all intermediate filaments is a pair of filamentous protein subunits wrapped around each other in a “coiled coil” structure. These pairs of proteins then line up end-to-end with other pairs to create long fibrous proteins. Multiple fibrous proteins coil around each other to create the intermediate filament (Figure 4.20). The coiled coil subunits of intermediate filaments possess an antiparallel arrangement, so there is no polarity (no + or – ends). This means that intermediate filaments cannot rapidly polymerize, as seen in actin, and have no role in cell movement. Their function is purely structural.
The structure of intermediate filaments allows them to bear tension well. Intermediate filaments are thus well-suited for maintaining a cell’s shape, and anchoring the nucleus and other organelles in place within the cytoplasm. These filaments are only found in animal cells, which do not have structural support from cell walls.
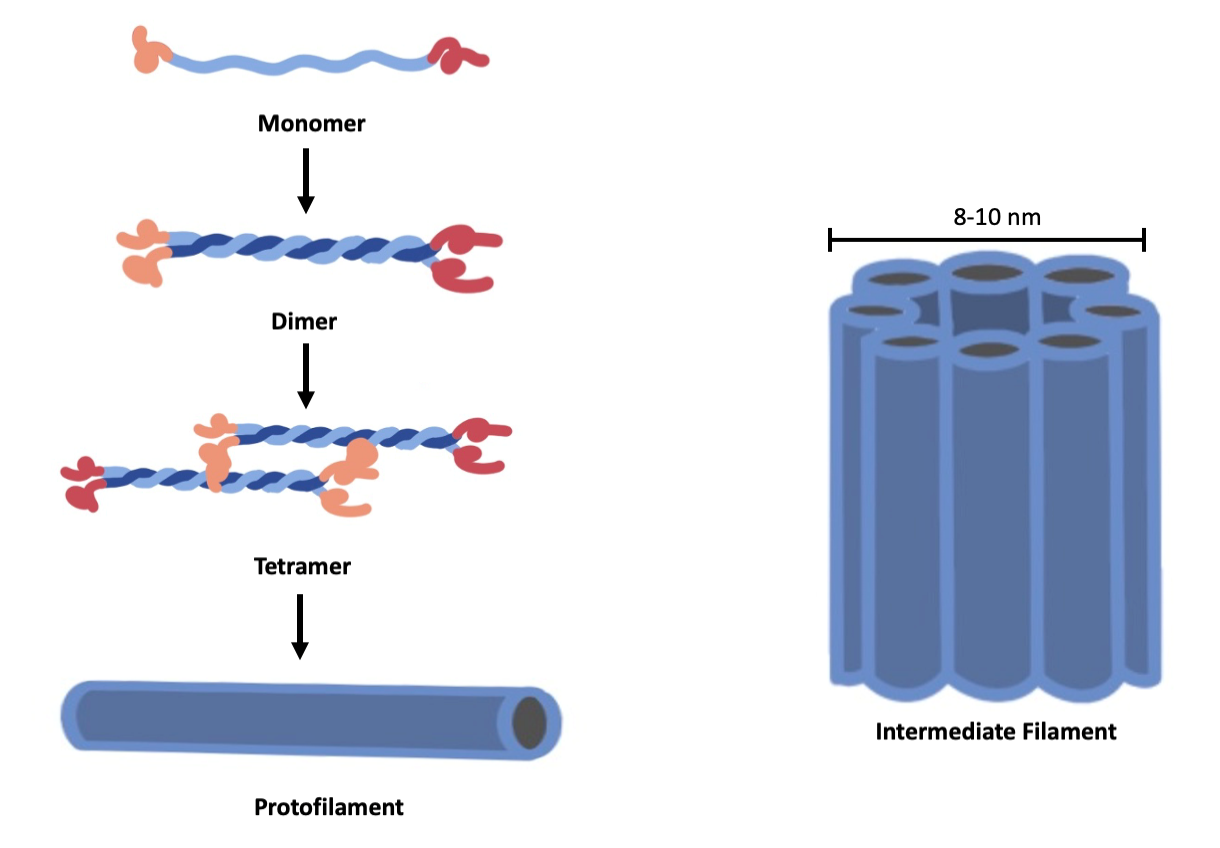
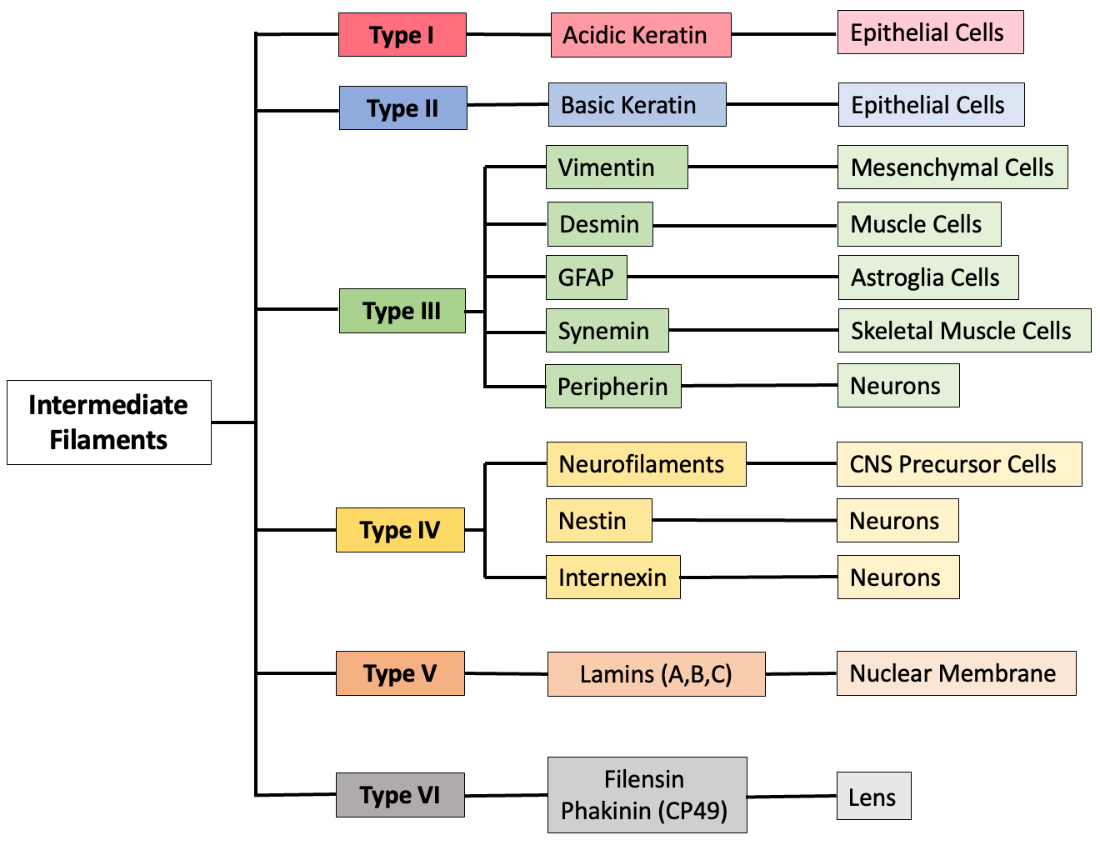
There are many types of intermediate filaments (Figure 4.21), each named after the protein they are made of. You are probably most familiar with keratin, the fibrous protein that strengthens your hair, nails, and the skin’s epidermis. This particular intermediate filament is made up of many copies of the protein called keratin. Some types of intermediate filaments, such as lamins, are found in all animal cells. Other types of intermediate filaments are found in specialized cell types; for example, neurofilaments are only found in neurons.
Microtubules
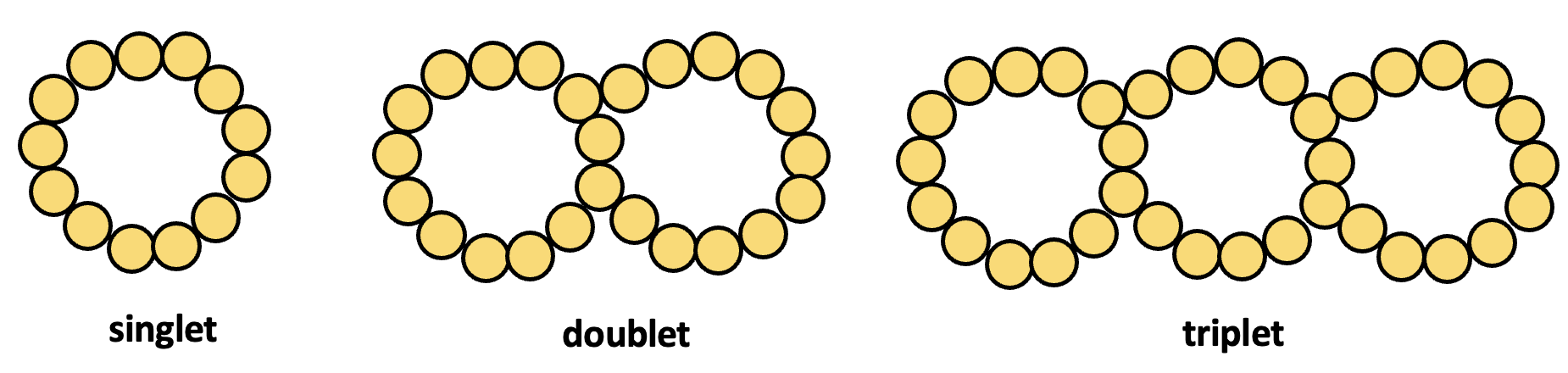
As their name implies, microtubules are small hollow tubes. Microtubules are made of two different globular proteins: α-tubulin and β-tubulin. Polymerized dimers of α-tubulin and β-tubulin, two globular proteins, comprise the microtubule’s walls (Figure 4.22). Like in microfilaments, a plus (+) and minus (–) end are formed, giving the microtubules polarity (but not electrical charge). Dimers can be added to either end, but are added more efficiently to the + end. The dimers assemble in a spiral formation that layers on top of the + end of the tube. As the tube assembles, it creates a vertical repeating pattern of β-tubulin and α-tubulin which are referred to as protofilaments. Each microtubule is typically comprised of 13 protofilaments, bring the diameter to about 25 nm. However, one microtubule can bind to a second microtubule, typically consisting of 10 protofilaments to create a doublet. Moreover, a doublet can bind to a third microtubule, typically consisting of 10 protofilaments to create a triplet (Figure 4.23).
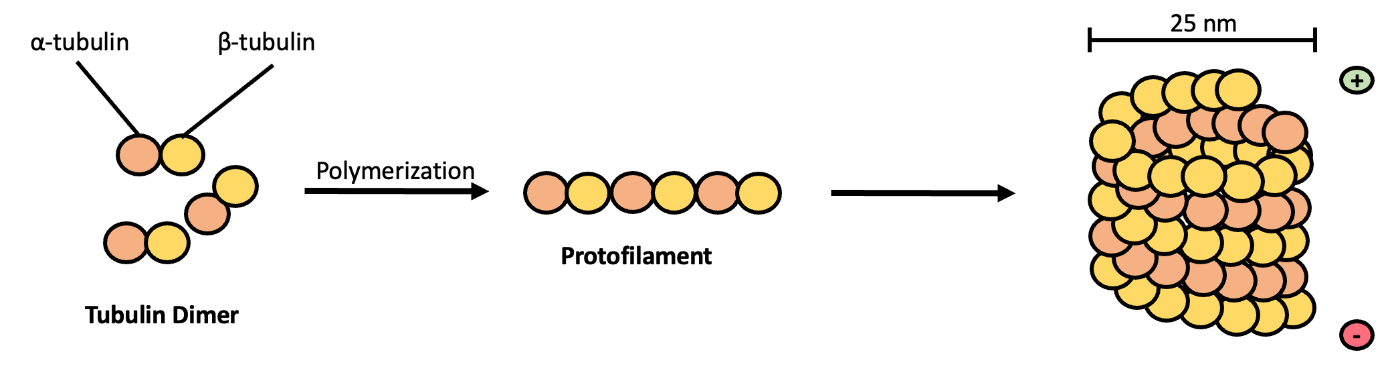
Like microfilaments, microtubules can assemble and dissemble to facilitate movement. These processes are regulated through microtubule associated proteins (MAPs) and GTP hydrolysis. GTP (guanosine triphosphate) is similar to ATP: cells can use it as an energy source. Both α-tubulin and β-tubulin monomers bind to GTP. α-tubulin is always bound to GTP, however β-tubulin can hydrolyze GTP to GDP. GTP-bound β-tubulin is more stable than GDP-bound β-tubulin, so GTP-bound tubulin is what gets added to the + end of the microtubule, usually with assistance from MAPs. However, GTP bound to β-tubulin will eventually hydrolyse, causing microtubules to disassemble at the – end. If the rate of hydrolysis is faster than the rate of addition by MAPs, then the microtubule will shrink. However, if the rate of MAP-mediated addition is faster than the rate of hydrolysis the microtubule will grow (Figure 4.24).
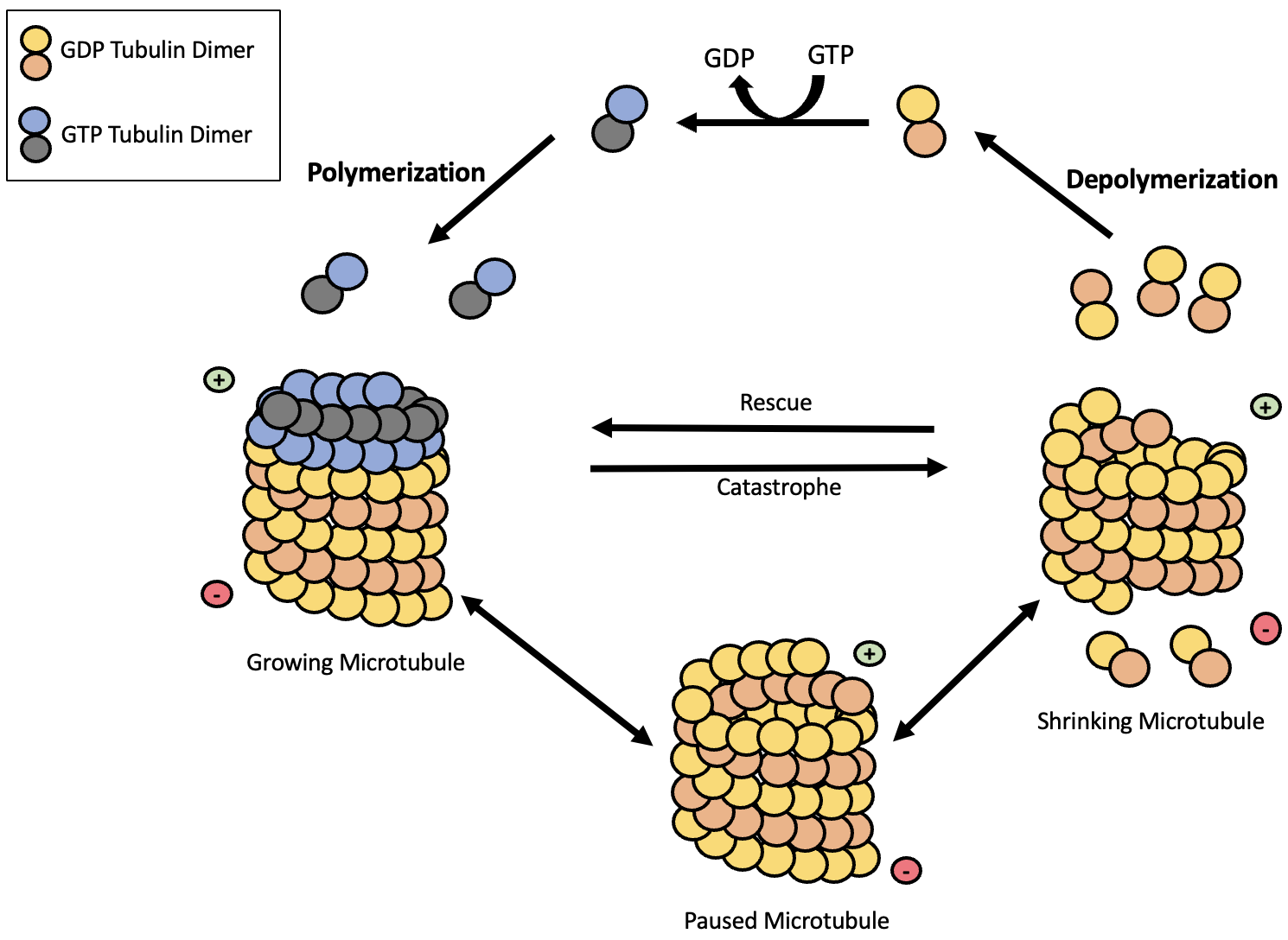
Microtubule assembly is quite slow, so they are often assembled at microtubule organizing centers. These organizing centers contain monomers of gamma-tubulin (γ-tubulin), which bind to accessory proteins and form a gamma-tubulin ring complex. The gamma-tubulin ring complex then binds to the – end of a microtubule, so that assembly can only occur at the + end of the microtubule. Two examples of microtubule organizing centers include centrosomes (see Chapter 4.2), which are located the nucleus of animal cells, and basal bodies, which form the base of cilia and flagella (see next subsection) near the cell membrane in some cells.
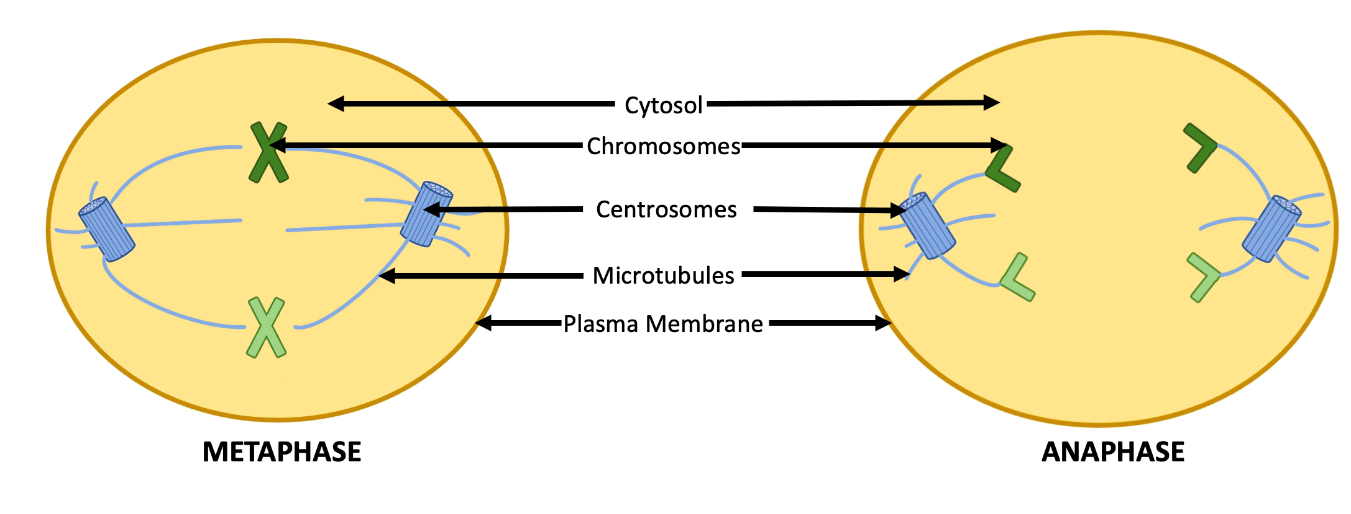
Microtubules have many functions within the cell including resisting compression, movement of substances within cells, and movement of whole cells. One of the substances moved by microtubules are chromosomes. For example, the centromeres mentioned above are responsible for arranging microtubules into a spindle during animal cell mitosis and meiosis. These spindle microtubules attach to chromosomes. When the microtubules grow, this moves the chromosomes to the centre of the cell (metaphase). The microtubules then shorten, in order to pull the chromosomes or sister chromatids to opposite ends of the cells (anaphase) (Figure 4.25). Microtubules can also facilitate movement of other substances within cells, such as vesicles and organelles. To do this, microtubules need the help of two motor proteins, dynein and kinesin. With some energy from ATP hydrolysis, dynein and kinesin can “walk” along microtubules, using the microtubules like roads or train tracks. For example, one end of a kinesin may be attached to a vesicle, while the other end walks along a microtubule, thereby moving the vesicle within the cell. This type of movement is critical for movement of substances within the endomembrane systems (Chapter 4.3). Microtubules facilitate the movement of whole cells through their function in cilia and flagella, as discussed below.
Link to Learning
See What is Kinesin? Ron Vale Explains for more on kinesin movement along microtubules
Flagella and Cilia
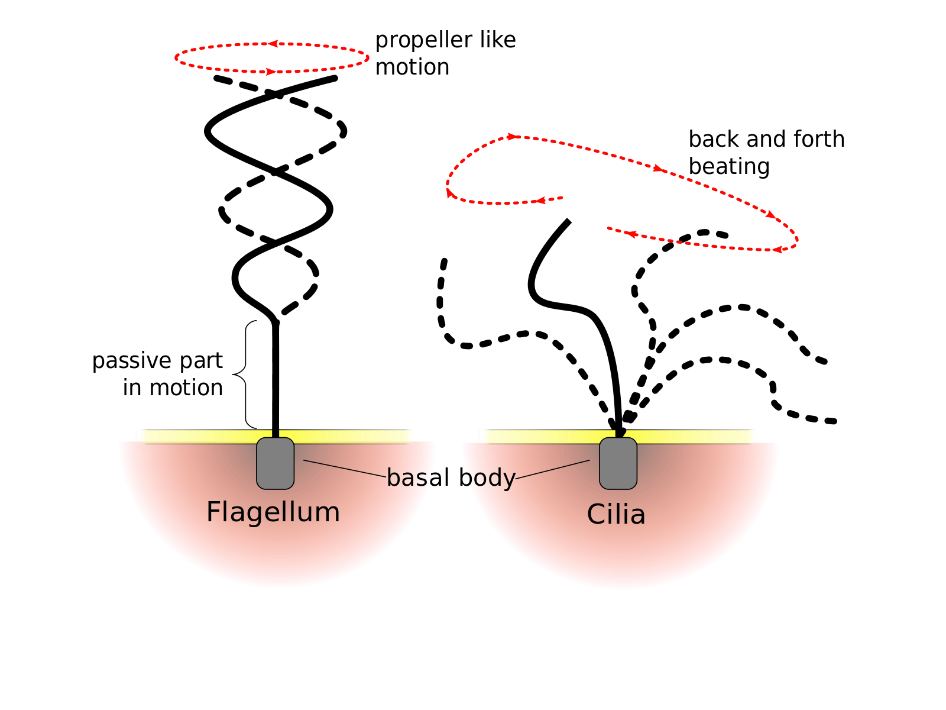
Flagella (singular = flagellum) and cilia (singular = cilium) are both hair-like extensions on a cell surface that are composed of microtubules and motor proteins. Flagella are long (10 – 200 µm long) tails on some motile cells, such as sperm from many species, and some protists like Euglena. Note that some prokaryotes have one or more flagella, but prokaryotic flagellum structure is different from eukaryotic flagellum structure. Each flagellum undulates (moves in a wave-like fashion) to create movement in a motor like fashion (Figure 4.26). Cilia are shorter (2 – 20 µm long), and (when present) there are often many of them extend along the plasma membrane’s entire surface. Cilia move in a back and forth beating motion (Figure 4.26). Together, these cilia movements can propel entire cells, such as some protists like Paramecium. When cells are stationary (i.e., most cells in multicellular organisms), the movement of cilia can cause other substances to move past the cell. For example, the cilia of cells lining human Fallopian tubes help move the ovum toward the uterus, and the cilia lining the cells of the human respiratory tract help move mucus up and toward the nostrils.
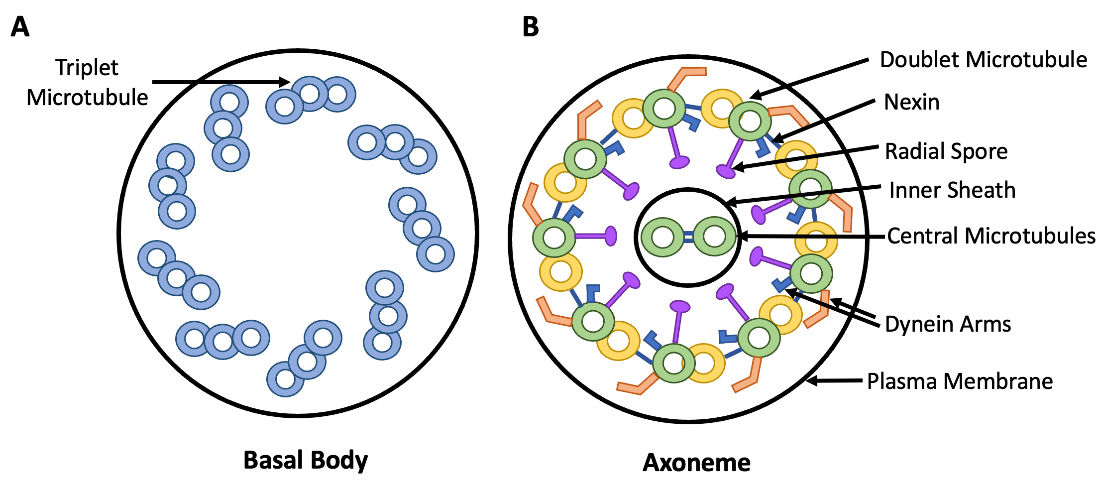
Although eukaryotic cilia and flagella are different in length, they have the same underlying structure. Each flagellum or cilium is anchored by a basal body (microtubule organizing centre). This basal body is located near the cell surface and is composed of nine microtubule triplets arranged in a circle (Figures 4.27A). Extending outward from the basal body is a structure called the axoneme. Each axoneme is about 0.25 µm in diameter, and the length varies (short for cilia, long for flagella). The axoneme is comprised of nine microtubule doublets arranged in a circle, with two singlet microtubules in the center, a configuration known as a “9 + 2” arrangement (Figure 4.27B). Refer back to Figure 4.23 for a reminder of singlet, doublet, and triplet microtubule structures. Dynein motor proteins exist along the entire length of each microtubule doublet in the axoneme. These dyneins form links between adjacent microtubule doublets, and use energy from ATP hydrolysis to help the axoneme bend. These axoneme bending creates motions described in the previous paragraph. Note that although cilia and flagella extend outward from a cell’s surface, each cilium and flagellum is still part of the cytoplasm and interior to the cell membrane. The cell membrane thus extends outward from the cell surface to keep the cilium or flagellum enclosed within the cytoplasm.
Visualizing the Cytoskeleton
The small diameter (6-25nm) of cytoskeletal structures makes them challenging to see using light microscopy. One technique we can use to view the cytoskeleton is staining it with fluorescent molecules or dyes. We will discuss two staining methods, one specific to filamentous (F)-actin (found in microfilaments), and one that can be used for any specific protein (e.g., tubulin and keratin).
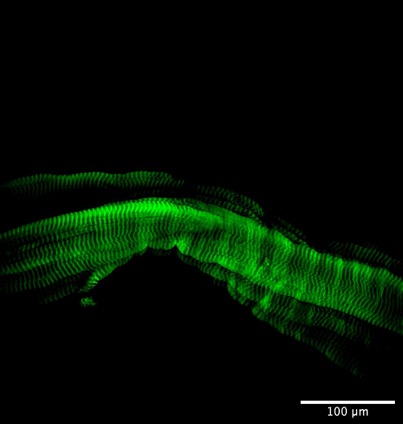
Phalloidin is a molecule that binds very specifically to F-actin. Phalloidin can actually act as a toxin (and is indeed produced by the highly toxic death cap mushroom, Amanita phalloides) because it prevents actin from depolymerizing. However, phalloidin is also a useful research tool for visualizing microfilaments because it only binds to F-actin, not G-actin. Phalloidin is not fluorescent, but chemists can attach fluorescent molecules to phalloidin. If fluorescently-labelled phalloidin is added to a cell, researchers can then visualize the location of F-actin using fluorescence or confocal microscopy. For example, the arrangement of F-actin in muscle cells can be seen in Figure 4.28.
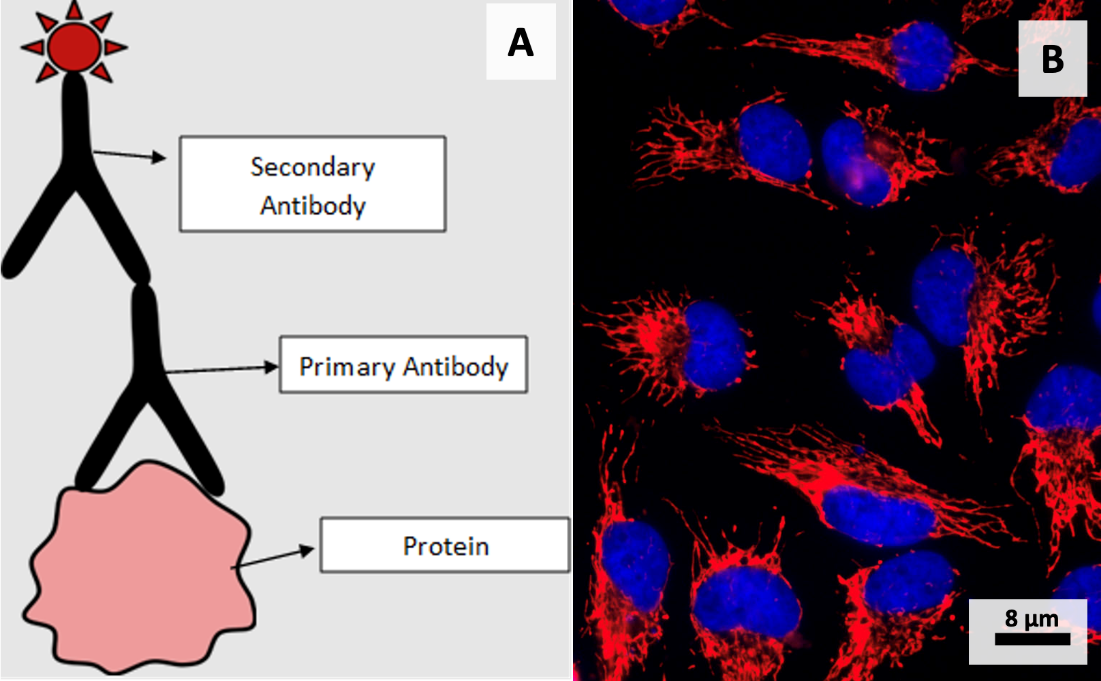
Immunofluorescence is another technique that can be used to visualize the cytoskeleton. This technique is called immunofluorescence because it uses antibodies, which are proteins used in the immune system of many animals. Antibodies are highly specialized: each type of antibody only binds to one type of target molecule (called an antigen), or a very small number of molecules with similar structure. Thus, if an antibody exists that can recognize a particular cytoskeletal protein (e.g., α-tubulin), we can use it for immunofluorescence. In this technique, a cell or tissue sample is first incubated with a primary antibody which binds to its target protein. This primary antibody is not fluorescent, nor does it bind in very high concentrations. However, we can add a secondary antibody to the sample that binds to the primary antibody, usually in higher abundance than the primary antibody. This secondary antibody has a fluorophore molecule attached to it, which concentrates a fluorescent signal close to the target protein (e.g., α-tubulin). The sample can then be imaged using fluorescence microscopy or confocal microscopy (Figure 4.29).