14.3 Degradation and Replacement of Damaged Macromolecules [in progress]
Key Concepts
By the end of this section, you will be able to do the following:
- Describe which types of stress can cause sufficient damage to macromolecules and organelles that they need to be degraded
- Describe mechanisms to degrade damaged proteins and organelles
- List processes that can be used to replace damaged proteins and organelles
Under extreme stress, macromolecules such as proteins, lipids, and DNA can be permanently damaged. Organelles may also be damaged, depending on the nature of the stress. If macromolecules and/or organelles are damaged beyond repair, the stress response will often involve degrading these damaged components and replace them, although not everything that is damaged can be replaced. For example, it is difficult to replace DNA and membranes (composed of lipids) that have experienced irreparable damage; damage to these cellular components usually lead to cell death (Chapter 14.5) if the damage cannot be repaired (Chapter 14.2). In this chapter, we focus on the degradation of proteins and organelles, which can often be replaced as long as the cell has sufficient resources to do so. The two types of degradation are proteasomal degradation (degrades proteins) and autophagy (degrades many types of macromolecules and organelles). The products of degradation can then be used to synthesize replacements.
Proteasomal degradation
[potential to expand the details]
Most kinds of stress can cause proteins can become denatured and aggregated. For example, extreme heat, extreme cold, osmotic stress, and oxidative stress can all cause irreparable protein damage. These damaged and aggregated proteins are non-functional and potentially toxic to the cell. Instead of inducing cell death, the cell can degrade specific proteins and synthesize new ones. Proteins to be degraded are tagged with ubiquitin and then degraded by proteasomes (Figure 14.10). Proteasomes are large protease and ATPase complexes that degrade tagged proteins into small peptides using ATP (Figure 14.10). The peptides can then be further broken down into amino acids by other processes, and those amino acids can then be used in the synthesis of new proteins for the cell.
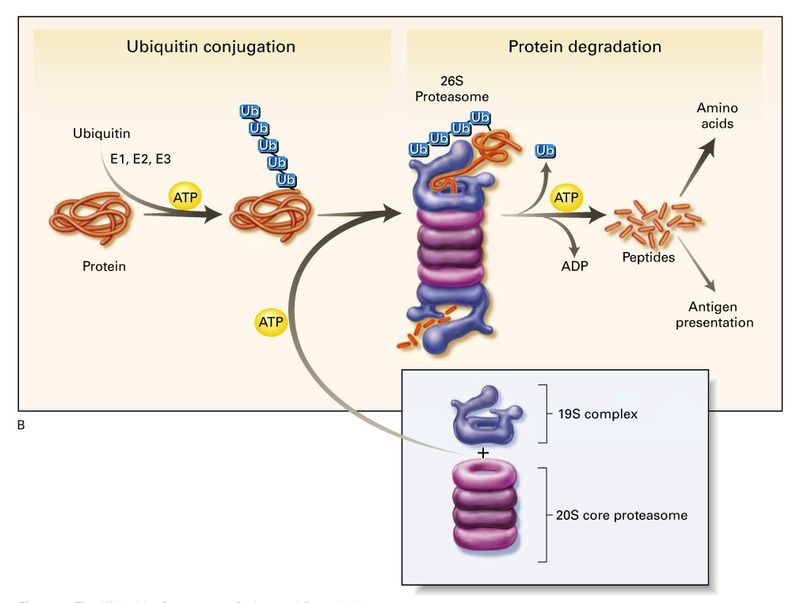
Figure 14.10 Ubiquitin tags irreparable proteins to be degraded in amino acids through proteasomal degradations. (Image: https://www.nejm.org/doi/10.1056/NEJM199612193352507) [copyright – likely have to replace]
Autophagy
Autophagy (self-eating) is a natural, self-degrative process that cells perform to remove damaged cell organelles like mitochondria and peroxisomes as well as old/damaged proteins. This process relieves the cell from various stress conditions such as starvation and cell damage. Autophagy can be selective or non-selective when removing damaged organelles and protein aggregates. There are two types of autophagy conserved in eukaryotes: microautophagy and macroautophagy (Figure 14.11). Even though they have distinct morphological features, they all lead to the delivery of cargo to a lytic organelle (a vacuole in fungi and plants or lysosome in animals) for degradation and recycling.
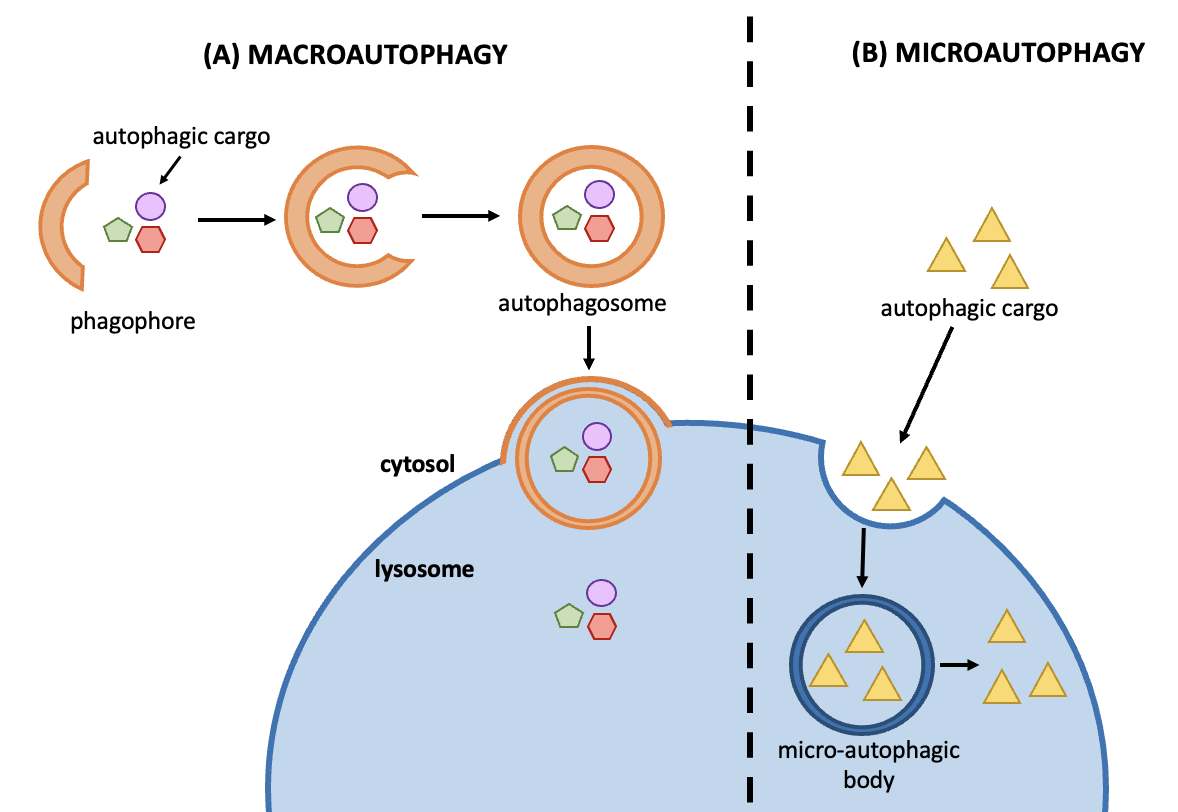
Figure 14.11 Overview of steps involved in macroautophagy and microautophagy. The lysosome depicted here is much larger than autophagosome in yeast and in plants, but not in mammals.
Link to Learning
Some more information about autophagy: https://www.youtube.com/watch?v=tf8sSome1lE
Macroautophagy
The macroautophagy process (Figure 14.11A) helps cells to degrade and recycle their cytoplasm in an evolutionarily-conserved manner. During macroautophagy, the cargo (material destined for degradation) is surrounded by an autophagosome, a sphere bound by two membranes. Autophagosome formation is regulated by Atg (autophagy) proteins. Upon completion of the autophagosome, the outer membrane fuses with a lytic organelle: a vacuole (in fungi and plants) or lysosome (in animals). This organelle contains acid hydrolase enzymes that will break down the inner membrane from the autophagosome and the autophagic cargo. The final step involves releasing of smaller molecules (products of the degradation) back into the cytosol for catabolic or anabolic metabolism.
Macroautophagy can be selective or non-selective. In non-selective macroautophagy, random portions of the cytoplasm are engulfed into autophagosomes and then transported to the lytic organelle. In selective macroautophagy, a particular cargo, protein complex, organelle, or microbe, is recognized and degraded. One example of selective macroautophagy is mitophagy, which refers to the autophagy of mitochondria (Figure 14.12). When a mitochondrion become damaged, a protein called PINK1 accumulates on the surface of the organelle, which interacts with a protein called Parkin. These proteins, along with others, “tag” the mitochondrion for destruction, resulting in the recruitment of autophagosome membranes (Figure 14.12)
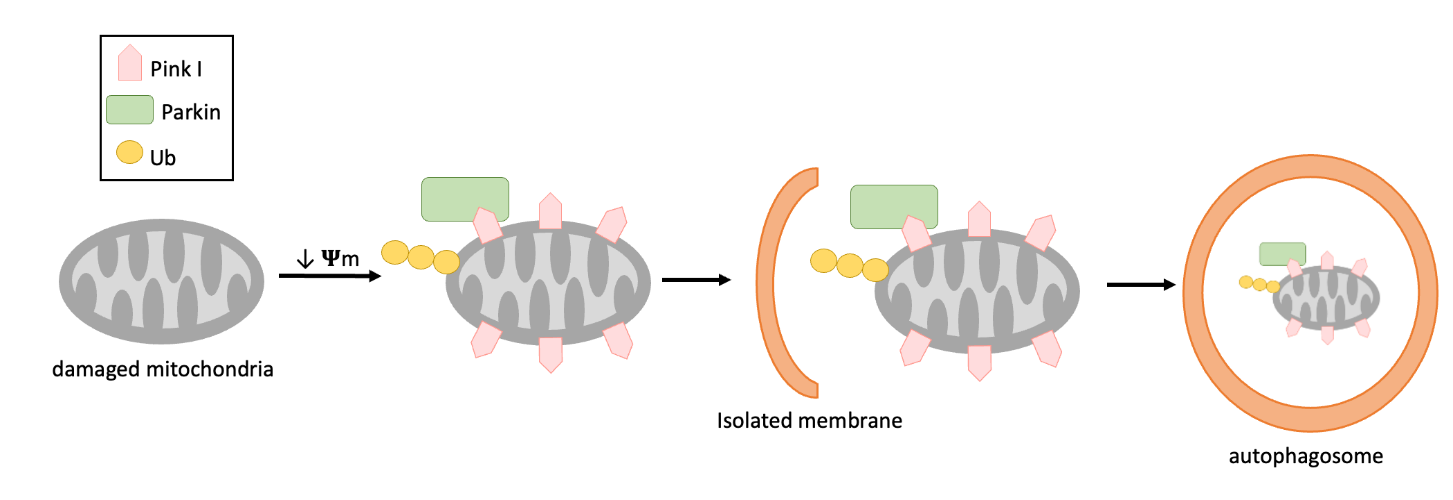
Figure 14.12 Process of mitophagy pathway in PINK1/ Parkin. Where ψm, mitochondrial membrane potential; PTEN-induced kinase 1, PINK1, Ub, ubiquitin.
Microautophagy
Microautophagy (Figure 14.11B) involves the direct engulfment of (non-specific) cytoplasmic cargo into the lysosome or other lytic organelle. It is a non-selective degradative process. The membrane of the lytic organelle first invaginates and forms a space that sequesters cytoplasmic material. The invaginated membrane is released into the organelle lumen as a microautophagic body (Figure 14.11B). Here, the vesicle closure is facilitated by ESCRT (endosomal sorting complex required for transport) proteins. The vesicle and its contents inside the lytic organelle are then degraded by acid hydrolases. This process can be independent of the core macroautophagy proteins (e.g., the Atg proteins).
Synthesis of new macromolecules and organelles
Once macromolecules and organelles are degraded, new ones must be synthesized to replace them in order to keep the cell functional. To conduct protein synthesis, cells must transcribe the mRNA from the gene encoding the protein, and then translate the mRNA to a polypeptide using ribosomes, amino acids, and tRNA. Ribosomes may synthesize these new proteins in the cytosol or on the rough endoplasmic reticulum. This protein synthesis requires lots of ATP (energy) and often requires molecular chaperones to ensure the new polypeptides are correctly folded. Another process crucial for the assembly of organelles is lipid synthesis (lipogenesis), which occurs in the smooth endoplasmic reticulum. This generates new phospholipids to incorporate into the membranes of new organelles. Lipogenesis also requires a large amount of ATP to proceed. The organelle synthesis process varies among organelle types. For example, new mitochondria are made via growth and binary fission of existing mitochondria, while new lysosomes are formed from membranes in the endomembrane system.

