14.2 Repair of Damaged Macromolecules [in progress]
Key Concepts
By the end of this section, you will be able to do the following:
- Describe which types of stress can cause mild damage to macromolecules
- Explain how mild damage to macromolecules can be repaired
Macromolecules and organelles can be damaged in various ways by exposure to different stressors. If only mild stress occurs, there is the possibility of repairing the damaged macromolecules or organelles. Mild stress is any stressor that causes the cell to experience conditions that are outside of its homeostatic range for a given condition, but is unlikely to cause permanent or irreparable damage to macromolecules (e.g., DNA, lipids, proteins) and cellular components (e.g., membranes). This section discusses what kinds of stress can cause mild damage to each of these macromolecules and how the damage can be repaired.
DNA repair
There are many mechanisms to repair mild DNA damage (Figure 14.2). One is base excision repair, which involves removing a single damaged base and replacing it with a new one. First, a DNA glycosylase enzyme recognizes and removes the damaged base from the DNA strand. Once the damaged base has been removed, another couple of enzymes remove the rest of the nucleotide from the DNA strand. This leaves a gap in the strand. DNA polymerase fills this gap by adding a new, undamaged nucleotide, and DNA ligase seals the strand to make it continuous again (Figure 14.5).
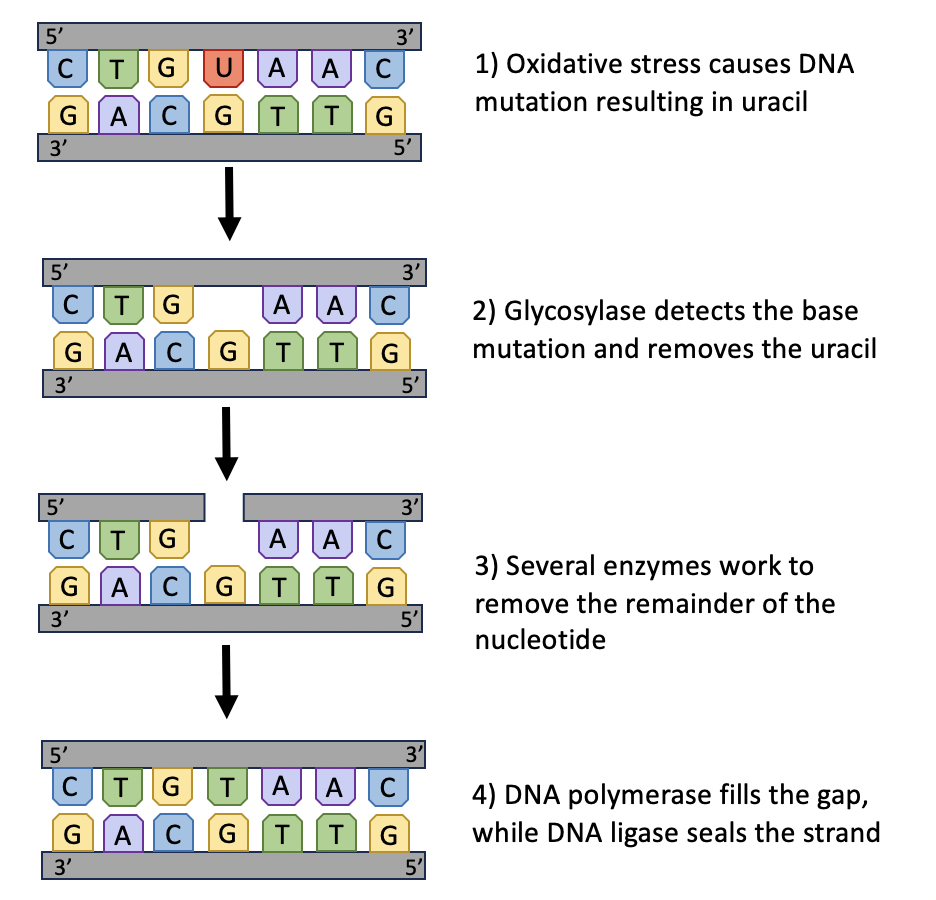
Figure 14.5. Base excision repair involves removing a single damaged base and replacing it with a new one. Here the replacement of an incorrect (uracil, U) with the correct base (thymine, T) is shown.
Lipid and membrane repair
Repair to damaged lipids and membranes is possible, but not very common. Mild lipid damage due to oxidative stress can be reversed by enzymes like peroxiredoxin (Figure 14.6). This can be an effective part of the stress response if the oxidative damage to lipids is mild, and has not substantially compromised the integrity of membranes.
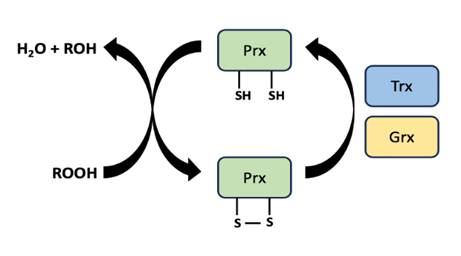
Figure 14.6. Oxidized lipids (represented by ROOH) can be reduced to their original form (represented by ROH) by enzymes such as peroxiredoxin (Prx), which aids in removing the excess oxygen molecules. Prx (an oxidized protein) is reduced via either Trx (thioredoxin) or Grx (glutaredoxin).
Damaged membranes (composed of phospholipids) that have small breaks in their membrane can be repaired in some cell types and can be repaired by different mechanisms that remove or replace the damaged membranes. However, if there are many breaks in the membrane then this mechanism will not be useful. An example of one mechanism to fix the breaks in membranes is patching (Figure 14.7). Patching is when several vesicles fuse to the membrane at the site of the breaks to replace the damaged or missing phospholipids with new ones (Figure 14.7).
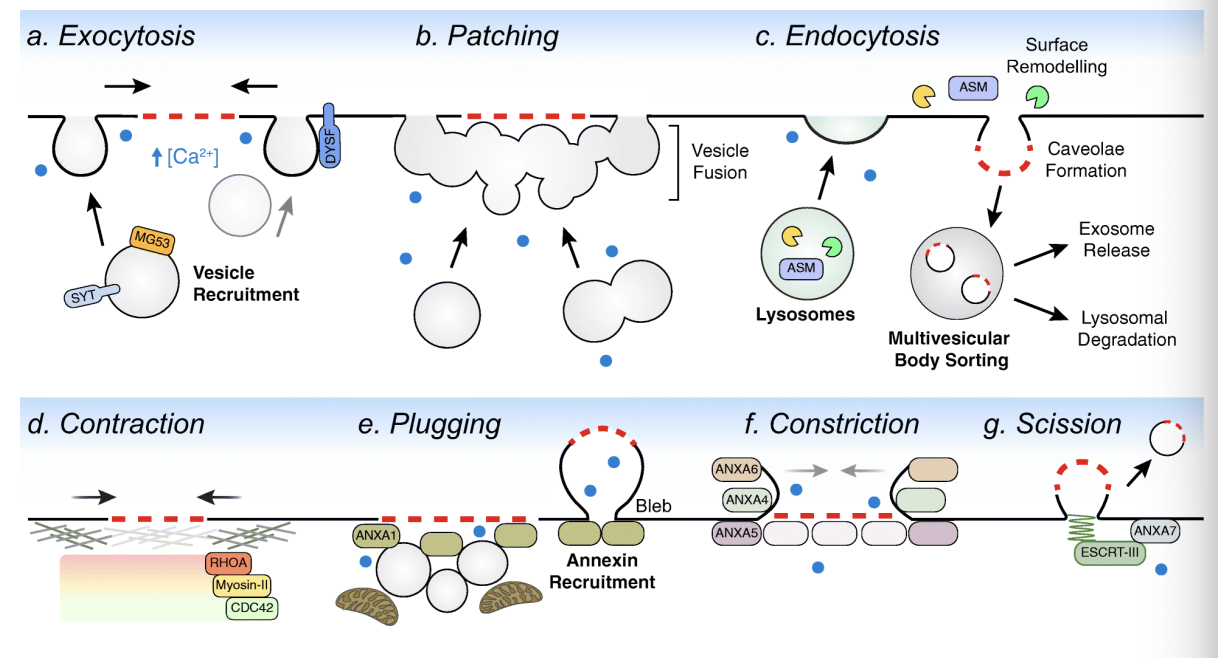
Figure 14.7. Mildly damaged membranes can be replaced or repaired via exocytosis, patching, endocytosis, contraction, plugging, constriction, and scission. [copyright – replace and/or just focus on patching?]
Protein repair
Denatured proteins can often be refolded, as long as the original amino acids are still intact, like in denaturation resulting from temperature or osmotic stress. Molecular chaperones like heat shock protein 70 (Hsp70) can refold proteins that have been partially denatured (Figure 14.8). However, it is challenging to repair or refold proteins that have aggregated. Damage to amino acids can be more difficult to repair. Some oxidation of amino acids (under oxidative stress) can be reversed by enzymes such as thioredoxin and glutaredoxin (Figure 14.9). However, if these chemical modifications to amino acids cause protein denaturation and aggregation, it is more difficult to repair the damage.
Figure 14.8 [To add – chaperone action]
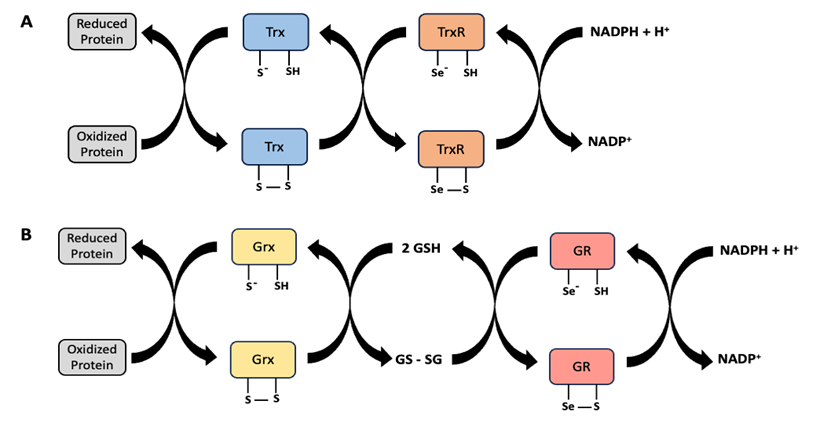
Figure 14.9 Oxidized amino acids in proteins can be reduced by enzymes in the (A) thioredoxin (Trx) and (B) glutaredoxin (Grx) family. Trx is reduced via oxidation of thioredoxin reductase (TrxR), followed by reduction of TrxR by NADPH. Grx is reduced by oxidization of glutathione (GSH) to GSSG, followed by reduction of GSSG via oxidation of glutathione reductase (GR), and reduction of GR by NADPH.

