12.3 Strategies and Mechanisms of Temperature Stress Tolerance
KEY CONCEPTS
By the end of this section, you will be able to do the following:
- Explain how the main strategies cells can use to combat temperature stress are effective at restoring cell homeostasis.
- Give examples of two mechanisms that can be used to combat mild heat or cold stress, and organisms that use those mechanisms.
- Give one example of a mechanism that can be used to combat extreme temperature stress.
Environmental temperature is constantly changing, and cells must be able to tolerate the cellular changes associated temperature fluctuations. In addition, most organisms do not generate internal body heat (except for mammals and birds), so the temperature of their cells/bodies normally follows that of their external environment. This section describes how cells respond to both mild and extreme heat and cold. Note that this section does not consider freezing temperatures; response to freezing stress is discussed in Chapter 13.
Responding to Mild Heat or Cold Stress
Strategy 1: Alter Macromolecule Composition
Given that temperature impacts the flexibility of proteins and the fluidity of membranes, cells can adjust the composition of their proteins and membranes to maintain optimal flexibility/fluidity under mild temperature stress. This can be accomplished by synthesizing warm- or cold-adapted proteins, changing the composition of phospholipids in membranes, and increasing cholesterol content in membranes.
The amino acid composition of a protein influences its flexibility, so cells can synthesize different versions of proteins, called protein isoforms, that counteract the effects of temperature on protein flexibility. Protein isoforms are proteins that are highly similar to each other in structure and perform similar or identical roles within cells. They often originate from a single gene or gene family. Under temperature stress, cells can produce cold-adapted or warm-adapted protein isoforms, which have a combination of structural and functional adaptations that allow them to perform the same function as each other at cold or warm temperatures, respectively. For example, cold-adapted proteins have a different amino acid composition which increases the number of small and flexible amino acids, such as glycine and proline. This increases the protein’s overall flexibility and allows the protein to maintain its function in cold conditions. Conversely, warm-adapted proteins need help staying stable at high temperatures. For example, the Archaean species Pyrolobus fumarii (Figure 12.2B) has warm-adapted proteins with an increased number of ionic interactions, large hydrophobic residues, and disulfide bonds to help them maintain their structure and function at high temperatures.
Cells can counteract the effects of temperature on membrane fluidity by modifying membrane composition. As described in Chapter 5.1, phospholipid structures can vary in ways that impact their fluidity. For example, phospholipids have the highest fluidity when their fatty acid tails are short and unsaturated (Figure 12.11A), and their head groups promote a conical shape (e.g., ethanolamine) (Figure 12.11B). Conversely, phospholipids have the lowest fluidity when their fatty acid tails are long and saturated (Figure 12.11A), and their head groups promote a cylindrical shape (e.g., choline) (Figure 12.11B). Cells under mild cold stress can therefore modify membrane composition by increasing the proportion of phospholipids with short, unsaturated fatty acids and/or head groups that promote a conical phospholipid shape. Conversely, cells under mild heat stress can do the opposite: increasing the proportion of phospholipids with long, saturated fatty acids and/or head groups that promote a cylindrical phospholipid shape.
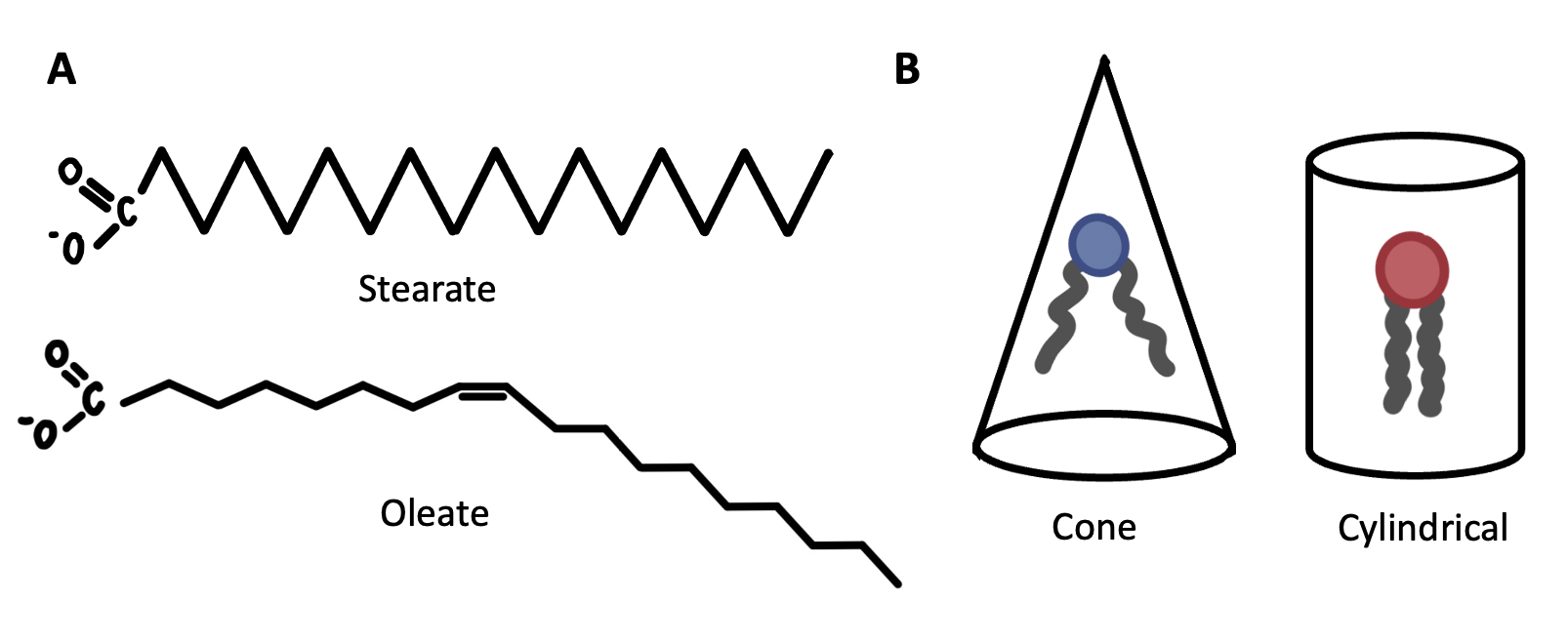
Cholesterol is a lipid that can be inserted into cellular membranes to prevent them from becoming too rigid or too fluid. Recall from Chapter 5.1 that cholesterol is a series of hydrocarbon rings, making it a very rigid and bulky molecule. At warm temperatures, such as at human body temperature, cholesterol decreases membrane fluidity because of its rigid structure. At low temperatures, the rigid structure of cholesterol causes it to act as a “spacer” that prevents the phospholipids from packing too closely together, causing membrane fluidity to increase. Thus, a cell can modify both its phospholipid and cholesterol content to decrease or increase membrane fluidity.
Strategy 2: Modify Metabolism to Accommodate Lower ATP Availability
As described in Chapter 12.2 mild heat and mild cold can both decrease the rate of catalysis via enzymes, which slows ATP production. See Chapter 11.3 for information on how cells can adjust their metabolism to deal with this energy limitation, either by decreasing energy demand or increasing available energy.
Responding to Extreme Heat or Cold Stress
As explained in Chapter 12.2, extreme temperatures can have detrimental effects on macromolecules which may be temporary or permanent. Cells may employ mechanisms to repair or replace damaged macromolecules. If the damage is too severe, the cell may have to undergo cell death. Chapter 14 addresses various mechanisms cells and organisms can use to respond to these challenges.
One example of how cells repair temperature-damaged proteins is by using heat shock proteins (HSPs), which are found in almost every organism. A major effect of extreme temperatures on macromolecules is protein denaturation and aggregation. HSPs are produced in response to high temperatures and act as molecular chaperones, meaning they help other proteins fold correctly and maintain their proper shape. They also help prevent protein aggregation and can target damaged proteins for degradation by the cellular machinery. Essentially, HSPs can reverse the process of denaturation shown in Figure 12.4.

